Research Article / Open Access
DOI:10.31488/bjg.1000132
Clinical Outcomes Correlating to a One-Day Shift in Sustained Feeding Tolerance in Very Low Birth Weight Infants in the ‘Connection Trial’
Josef Neu, MD1, Teresa Del Moral, MD2, Jenelle Ferry, MD3, Scott O Guthrie, MD4, Andrea Nagy, MD5, Ajay Talati, MD6, Marcus Thuresson7, Anders Kronström7, Staffan Strömberg7, Jonas Rastad*7
1. UF Health Shands Children’s Hospital, Gainesville, FL, USA
2. University of Miami, Miami, FL, USA
3. St. Joseph's Women's Hospital, Tampa, FL, USA
4. Vanderbilt University School of Medicine, Nashville, TN., and Jackson-Madison County General Hospital, Jackson, TN, USA
5. University of Debrecen Institute of Pediatrics, Debrecen, Hungary
6. The University of Tennessee Health Science Center, Memphis, TN, USA
7. Infant Bacterial Therapeutics AB, Stockholm, Sweden
*Corresponding author: Jonas Rastad, Infant Bacterial Therapeutics, Bryggargatan 10, SE-11121 Stockholm, Sweden
Abstract
Introduction. Validated endpoints for a sustained feeding tolerance (SFT) are lacking for premature infants despite growth being a key success factor of neonatal care. Material. The Connection trial is a phase 3 study of the pharmaceutical grade probiotic IBP- 9414 (L. reuteri) with a primary endpoint of the time to SFT in infants of a birth weight (BW) of 500-1500g randomized 1:1 to a daily enteral dose of IBP-9414 (109 CFU) or placebo from within 48 hours of birth till a corrected gestational age (GA) of 34 weeks+6 days. A treatment- blind analysis of clinical correlates to SFT was performed for the first 319 infants with a BW of 750-1000g. SFT was defined as the first day of enteral feeds ≥120ml/kg body weight maintained for ≥10 consecutive days (10-day definition) or until study end (study-end definition) in combination with no parenteral nutrition and a mean body weight increase of ≥10 g/kg/day for the respective duration. Clinical outcomes associated with a one-day shift in the time to SFT were evaluated with regression models. Results. SFT with the 10-day and study-end definitions was reached by 248 and 216 infants at mean 17.2 days (Sd 10.4 days) and 24.6 days (Sd 15.4 days), respectively. Clinical outcomes correlated the strongest to a one-day shift in time to SFT with the 10-day definition. These correlations included events of NEC confirmed by independent adjudication of abdominal X-rays (p=0.0002), gastrointestinal adverse events (AEs, p=0.0001), late onset sepsis (p=0.0001), clinically suspected sepsis (p=0.01), daily weight gain (p=0.0014), bronchopulmonary dysplasia (p=0.036), retinopathy of prematurity (p=0.0004), respiratory AEs (p<0.0001), duration of hospitalization (p<0.0001) and days with systemic antibiotic medication (p<0.0001). Conclusion. This treatment-blind evaluation of extremely low BW infants introduces a strict definition of SFT and underlines the clinical relevance of a one-day change in the time to SFT in premature infants.
Key words: premature infants, enteral feeding, feeding tolerance, endpoint validation
Introduction
Enteral feeding is key to the optimal growth, maturation and development of premature infants [1-3]. Especially in the extremely low birth weight (ELBW) infants there is controversy as regards the most favorable age at which enteral feeding should commence and the speed by which the feeding volumes should increase [4,5]. A host of factors may interfere with the intent to progress these volumes based e.g., on the risks of inducing necrotizing enterocolitis (NEC) and clinical signs attributable to feeding intolerance [6,7]. There is, however, consensus that full enteral feeding should be reached early in order to ascertain optimal growth and reduce unwanted direct and indirect effects of a prolonged parenteral nutrition like cholestasis, late onset sepsis, increased use of systemic antibiotics and delayed gut maturation [5,8,9]. The definition of a full enteral feeding usually includes a daily feeding volume of at least 120-150 ml/ kg body weight, sometimes required to persist for a few days, or an absent need for support with parenteral nutrition [10-13]. Little, however, has been explored as to clinical outcomes correlating to a full enteral feeding sustained over longer periods of time and a relevant definition of such an endpoint in drug development trials in premature infants.
The ‘Connection Trial’ on IBP-9414 is the first and largest controlled trial in the preterm population on the effects of a live bacterium, Lactobacillus reuteri, as a pharmaceutical-grade ingredient (ClinicalTrials.gov Id: NCT03978000). The study is conducted under US IND and EU CTX in 10 countries. This quality control differentiates IBP-9414 from other probiotics, all of which are food additives with lesser quality control, including drug content and manufacturing process, as well as critical evaluation of risks and benefits of their use [14-16].
A primary endpoint of the ‘Connection Trial’ is the time to a sustained feeding tolerance (SFT). Utilizing reports from investigators in this trial, we herein demonstrate nature and strength of correlations for clinical outcomes to a one-day shift in the time to a full enteral feeding maintained over longer periods of time.
Material and Methods
The ‘connection trial’
The ‘Connection Trial’ is a phase 3, randomized placebo-controlled multicenter study in the US and EU, including Israel, on the safety and efficacy of IBP-9414 (Table 1). Infants of 500-1500 g birth weight are randomized 1:1 to a single daily enteral dose of IBP-9414 at 109 colony- forming units (CFU) or sterile water placebo from within 48 hrs. of birth till a corrected gestational age (cGA) of 34 weeks+6 days. The primary endpoints of the ‘Connection Trial’ are the incidence of NEC and the time to SFT. As a precaution, this phase 3 study was initiated in infants of 750-1000 g birth weight with subsequent extension of infant recruitment after independent safety evaluation.
Table 1.The ‘Connection trial’ at a brief (ClinicalTrials.gov Id: NCT03978000)
| Eligible patients: Infants with a birth weight of 500- 1500g and GA 23-32 w meeting the inclusion and exclusion criteria of the study. |
| Study design: Randomized, parallel group, multicenter phase 3 study designed to interfere as little as possible with clinical routines at participating units. |
| Primary Endpoints: Incidence of NEC and time to SFT. Secondary endpoints: Medical NEC, Surgical/autopsy NEC, all-cause mortality, weight gain, duration of hospitalization, feeding intolerance. |
| Study drug: IBP-9414 containing pharmaceutical grade L. Reuteri given orally or by enteral tube at a single daily dose of 109 CFU or placebo from within 48 hrs. of birth to 34 weeks +6 days GA. |
| Contribution centers: Neonatal Intensive Care Teams in Bulgaria, France, Hungary, Israel, Poland, Romania, Serbia, Spain, UK, and US. |
Sustained feeding tolerance
Due to the absence of validated definitions for SFT, options needed to be explored in order to define a clinically meaningful study endpoint. This was discussed with the relevant Health Authorities and a combination of an enteral feeding volume and weight gain, maintained during a period of time, was agreed. A procedure for validating SFT was adopted that involved analyzing the first circa 300 infants in the ‘Connection trial’ to unveil the extent of clinical correlates to a one-day shift in the time to SFT. This analysis was performed blindly, i.e., without knowledge of the assigned treatment group for the infants.
An expert panel consisting of three senior pediatricians, nurse and parent representatives were presented with available clinical outcomes for the correlation analysis (Table 2). Upon request they ranked the outcome categories in decreasing order of perceived clinical importance in the context of SFT. The strength of statistical correlations for these categories to a one-day shift in the time to SFT was analyzed for two alternative ways of defining SFT. The expert panel subsequently was presented with the data output with the expressed intent to conclude as to the clinical relevance of a one-day shift in the time to SFT and which definition they would recommend for use in the ‘Connection Trial’
Table 2.Output from the expert panel as to the ranked order of outcome categories potentially correlating to the time to SFT in decreasing order of perceived relevance
| Number of infants with a NEC event confirmed by independent evaluation of abdominal radiographs. |
| Number of days per infant with clinical signs of feeding intolerance as defined by investigators (e.g., vomiting, abdominal distention, bradycardia). |
| Number of infants with a relevant gastrointestinal AE (abdominal distention, colitis, diarrhea, enteritis, gastroenteritis, gastrointestinal hemorrhage, gastrointestinal necrosis, hematochezia, ileus, intestinal perforation, necrotizing colitis, necrotizing enterocolitis, peritonitis, pneumoperitoneum, vomiting). |
| Number of infants with late onset sepsis (blood culture positive >72 hrs. after birth). |
| Weight gain per infant (g/day from first dose of study drug till end of study). |
| Number of infants with clinically suspected sepsis (positive blood culture lacking). |
| Number of infants with bronchopulmonary dysplasia (including chronic respiratory disease/disorder). |
| Number of infants with retinopathy of prematurity. |
| Number of respiratory AEs per infant (anoxia, apnea, atelectasis, bronchopulmonary dysplasia, chronic respiratory failure bronchospasm, dyspnea, emphysema, hypercapnia, hypoventilation, hypoxia, immature respiratory system, lung disorder, pneumomediastinum, pulmonary hypertension, pulmonary oedema, pulmonary, pneumatocele, respiratory acidosis, respiratory alkalosis, respiratory arrest, respiratory depression, respiratory disorder, respiratory distress, respiratory failure, stridor, tachypnoea, upper respiratory tract congestion, alveolitis, bronchitis, bronchiolitis, pneumonia, respiratory tract infection. |
| Days of hospitalization per infant. |
| Number of SAEs per infant. |
| Days with antibiotic use (class intended for systemic use) per infant. |
| Number of infants with concurrent respiratory and cardiac AEs (apnea or hypoxia and bradycardia). |
AE: Adverse event of a serious or non-serious character; SAE: serious adverse event
SFT was defined as the first day of tolerating daily enteral feeds of at least 120 ml/kg body weight with no use of parenteral nutrition (i.e., amino acids or fat) and with at least 10g/kg in average daily body weight increase, all of which should be maintained either throughout the rest of the study (study-end definition) or for a minimum of 10 consecutive days (10-day definition). The 10-day period was suggested by the expert panel as a potentially relevant duration for defining SFT. The time to SFT was measured as the number of days from the first dose of study product to the first day of the SFT period.
Data on the clinical outcomes were collected from the first 319 infants of the ‘Connection Trial’ for the period from the first dose of study drug until the infant had reached a gestational age (GA) of 34 weeks + 6 days or to discharge, if earlier. The overall time period during which data was collected for each infant averaged 48 days (median, 52 days).
Statistics
Continuous outcomes were analyzed with regression models. For naturally continuous variables, linear regressions were performed with the time to SFT as an explanatory variable, birth weight as a further covariate, and the clinical variables as a response. The regression coefficient for this analysis provides an estimate of the change in clinical outcome expected from a 1-day change in the time to SFT. In addition to point estimates, 95% confidence intervals were calculated for each regression coefficient and the P value for testing the null hypothesis that the coefficient is zero. For dichotomous clinical outcomes, logistic regressions were performed; the explanatory variables being time to sustained feeding tolerance, whether sustained feeding tolerance was achieved or not as a binary variable, and birth weight. The resulting regression coefficients represent log odds changes associated with a 1-day change in the time to SFT. As with the continuous variables, 95% confidence intervals and P values were calculated. Negative binomial regressions were done similarly for count data: the regression coefficients in those cases represent log incidence rate ratios associated with a 1-day change in the time to SFT. Nominal confidence intervals and p-values are presented, and no adjustment for multiplicity has been performed.
Any relationship of the time to SFT was also explored against the cumulated number of outcomes for each infant in the outcome groups of NEC, relevant gastrointestinal adverse events (AEs), late onset sepsis, clinically suspected sepsis, bronchopulmonary dysplasia, retinopathy of prematurity, and concurrent respiratory and cardiac AEs.
Results
Descriptive statistics
There were 216 and 248 infants meeting the study-end and 10-day definitions of SFT and the average times to SFT in these infants were 24.6 and 17.2 days, respectively (Table 3). NEC was confirmed by independent adjudication of abdominal x-rays or surgery/autopsy in 6.0% and 8.1% of the infants, rather few days were recorded with feeding intolerance and the frequency of gastrointestinal adverse events (serious and non-serious AEs) was 19.9% and 22.2%, respectively. The infants gained weight at an average of 21.2 grams/day during the time in the study and antibiotics were given on average 12.8 days and 15.4 days, respectively. Culture-positive late onset sepsis was found in 10.6% to 11.75 % and clinically suspected sepsis in 6%, 7.3% of the infants. Respiratory AEs were reported in about ⅔ of the infants in both SFT-definition groups, bronchopulmonary dysplasia in about ⅓ and retinopathy of prematurity in about ¼. The infants were hospitalized for a mean of 74.2 and 75.8 days, respectively.
Table 3.Descriptive statistics on clinical outcomes for infants fulfilling each definition of SFT.
| Study-end definition n=216 | 10-day definition n=248 | |
|---|---|---|
| Days to Sustained Feeding Tolerance | ||
| Mean (Sd) | 24.6 (15.4) | 17.2 (10.4) |
| Median (IQR) | 22 (11-36) | 14 (10-22) |
| Confirmed NEC Events, n (%) | 13 (6.0) | 20 (8.1) |
| Days with Clinical Signs of Feeding Intolerance | ||
| Mean (Sd) | 4.9 (8.4) | 5.2 (9.0) |
| Median (IQR) | 1 (0-6) | 1 (0-7) |
| Relevant Gastrointestinal AEs, n (%) | 43 (19.9) | 55 (22.2) |
| Late Onset Sepsis, n (%) | 23 (10.6) | 29 (11.7) |
| Weight Gain (g/day) | ||
| Mean (Sd) | 21.2 (4.3) | 21.2 (4.2) |
| Median (IQR) | 21 (19-24) | 21 (19-24) |
| Clinically Suspected Sepsis, n (%) | 13 (6.0) | 18 (7.3) |
| Bronchopulmonary Dysplasia, n (%) | 74 (34.3) | 85 (34.3) |
| Retinopathy of Prematurity, n (%) | 51 (23.6) | 65 (26.2) |
| Number of Respiratory AEs | ||
| 0 | 80 (37.0) | 95 (38.3) |
| 1 | 71 (32.9) | 75 (30.2) |
| 2 | 38 (17.6) | 45 (18.1) |
| 3+ | 27 (12.5) | 33 (13.3) |
| Days of Hospitalization | ||
| Mean (Sd) | 74.2 (21.1) | 75.8 (23.2) |
| Median (IQR) | 77 (59-89) | 77 (60-90) |
| Number of SAEs | ||
| 0 | 185 (85.6) | 205 (82.7) |
| 1 | 16 (7.4) | 22 (8.9) |
| 2 | 7 (3.2) | 9 (3.6) |
| 3+ | 8 (3.7) | 12 (4.8) |
| Days with Antibiotic Use | ||
| Mean (Sd) | 12.8 (42.9) | 15.4 (51.8) |
| Median (IQR) | 3 (0-10) | 4 (0-11) |
| Concurrent Respiratory- Cardiac AEs, n (%) | 7 (3.2) | 9 (3.6) |
Sd= standard deviation, IQR= interquartile range
The time to SFT increased with the accumulated number of adverse events in both the 10-day and study- definitions (Figure 1A, B). There were trends for the highest number of events in the infants with a lower body weight and GA at birth.
Quantitative statistics
The statistical correlation to the evaluated clinical outcome categories generally were stronger for a one-day change of the time to SFT with the 10-day definition as compared to the study-end ditto (Tables 4 and 5). This discrepancy was particularly evident for the coupling to sepsis, both late onset and clinically suspected sepsis, and for the gastrointestinal AEs. Both definitions, however, correlated strongly to clinical outcomes as NEC, daily weight gain, retinopathy of prematurity, respiratory AEs, days of hospitalization and days with antibiotic use. The few registered days with feeding intolerance showed no statistically significant coupling to the one-day change in the time to either definition of SFT.
Discussion
Attainment of full enteral feeding is an important goal in the intensive care treatment of premature infants [5,9]. This study originated from interactions with Health Authorities indicating that drug development trials on feeding tolerances would benefit from exploring what the strength and nature of clinical correlates would be to a one-day shift in the time to a sustained feeding tolerance. Establishment of such correlations has not previously been evaluated partially because a consensus definition of sustained feeding tolerance has not been established [10,11,13]. Here we provide such a definition and analyze its strength against a panel of adverse outcomes occurring in the postnatal intensive care treatment of ELBW infants.
This analysis of clinical correlates to a one-day shift of the time to SFT includes a first subset of ELBW infants recruited into the ‘Connection Trial’. For reasons of study integrity, no information has been unveiled as to the allocation of infants into the study groups of a once-daily enteral dose of 109 CFU of the pharmaceutical grade preparation of L. Reuteri or placebo. In contrast to all currently available probiotics (cf. 14,15), IBP-9414 is of pharmaceutical grade with quality standards equivalent to all drug products. Manufacturing requires full pharmaceutical GMP (21 CFR Part 210/211) throughout the entire process from cell banking to final drug product, including rigorous controls of raw materials, ingredients and excipients. The quality requirements also include testing for absence of a range of specified organisms as potential contaminants, batch control and shelf-life determination as well as validated procedures for a correct dosing.
The ’Connection trial’ is a prospective, multicenter phase 3 study conducted in 10 countries. The study protocol is designed to minimize interference with local treatment regimens. Variation in the infant feeding protocols of importance to the time to SFT may thus have occurred. Moreover, the study protocol includes no specified biochemical or other tests beyond common routines, whereby the available data for the correlation analysis were limited. All AE outcomes were based on reports from the responsible investigator, but the diagnosis of NEC relied on the per protocol requirement of independent adjudication of abdominal radiographs or verification by surgery or autopsy.
The analysis of clinical correlates to the brief, one-day change of the time to SFT aims at comparing alternate definitions in SFT and at clarifying to what extent such a change in the time to SFT correlates with important clinical outcomes. Our definition of SFT included an enteral feeding volume of at least 120 ml/kg per day, absence of parenteral nutrition use (i.e., no use of amino acids or fat) and a mean daily increase in body weight of at least 10 g/kg. This definition was deliberately set as a lower limit for the identification of ‘full’ enteral feeding with knowledge that both greater feeding volumes and body weight increases may be aimed at in the nutritional optimization of the VLBW infant [10,11,13]. It should be emphasized, however, that by both definitions the infant must acquire at least 120 ml/kg for every consecutive day during the two periods of evaluated study time. Inclusion of this time requisite for defining SFT substantiates the stability of the attained level of enteral feeding, which was considered relevant to drug development trials.
Table 4.Result of regression analysis for the 216 infants reaching the study-end SFT definition
| Mean | N events | Estimate* | 95 % CI | p-value | |
|---|---|---|---|---|---|
| Confirmed NEC Events | 13 | 1.0759 | (1.0346-1.1189) | 0.0002 | |
| Days with Clinical Signs of Feeding Intolerance | 4.9 | - | 0.9972 | (0.9807-1.0140) | 0.7421 |
| Relevant Gastrointestinal AEs | - | 43 | 1.0155 | (0.9939-1.0375) | 0.1599 |
| Late Onset Sepsis | - | 23 | 1.0278 | (1.0002-1.0562) | 0.0487 |
| Weight Gain (g/day) | 21.2 | - | -0.0546 | (-0.0915--0.0178) | 0.0041 |
| Clinically Suspected Sepsis | - | 13 | 1.0338 | (0.9991-1.0698) | 0.0561 |
| Bronchopulmonary Dysplasia | - | 74 | 1.0277 | (1.0085-1.0472) | 0.0045 |
| Retinopathy of Prematurity | - | 51 | 1.0393 | (1.0175-1.0616) | 0.0004 |
| Number of Respiratory AEs | 1.1 | - | 1.0200 | (1.0118-1.0283) | < 0.0001 |
| Number of Days of Hospitalization | 74.2 | - | 0.4772 | (0.3073-0.6470) | < 0.0001 |
| Number of SAEs | 0.3 | - | 1.0364 | (1.0083-1.0652) | 0.0107 |
| Days with Antibiotic Use | 12.8 | - | 1.0350 | (1.0190-1.0513) | < 0.0001 |
| Concurrent Respiratory and Cardiac AEs | 7 | 0.9786 | (0.9238-1.0367) | 0.4626 |
* For linear regression the estimate is the linear slope, for logistic regression the estimate is the odds ratio, and for negative binomial regression the estimate is the incidence rate ratio, all associated with a one day increase of time to sustained feeding tolerance.
Table 5.Result of regression analysis for the 248 infants reaching the 10-day SFT definition
| Mean | N events | Estimate* | 95 % CI | p-value | |
|---|---|---|---|---|---|
| Confirmed NEC Events | 20 | 1.0765 | (1.0357-1.1189) | 0.0002 | |
| Days with Clinical Signs of Feeding Intolerance | 5.2 | - | 1.0029 | (0.9791-1.0272) | 0.8155 |
| Relevant Gastrointestinal AEs | - | 55 | 1.0586 | (1.0293-1.0887) | 0.0001 |
| Late Onset Sepsis | - | 29 | 1.0671 | (1.0326-1.1028) | 0.0001 |
| Weight Gain (g/day) | 21.2 | - | -0.0831 | (-0.1333--0.0328) | 0.0014 |
| Clinically Suspected Sepsis | - | 18 | 1.0531 | (1.0108-1.0972) | 0.0133 |
| Bronchopulmonary Dysplasia | - | 85 | 1.0275 | (1.0018-1.0539) | 0.0360 |
| Retinopathy of Prematurity | - | 65 | 1.0500 | (1.0221-1.0786) | 0.0004 |
| Number of Respiratory AEs | 1.2 | - | 1.0331 | (1.0214-1.0449) | < 0.0001 |
| Number of Days of Hospitalization | 75.8 | - | 0.7710 | (0.5086-1.0335) | < 0.0001 |
| Number of SAEs | 0.4 | - | 1.0420 | (1.0076-1.0776) | 0.0162 |
| Days with Antibiotic Use | 15.4 | - | 1.0585 | (1.0353-1.0821) | < 0.0001 |
| Concurrent Respiratory and Cardiac AEs | 9 | 1.0392 | (0.9848-1.0967) | 0.1611 |
*For linear regression the estimate is the linear slope, for logistic regression the estimate is the odds ratio, and for negative binomial regression the estimate is the incidence rate ratio, all associated with a one day increase of time to sustained feeding tolerance.
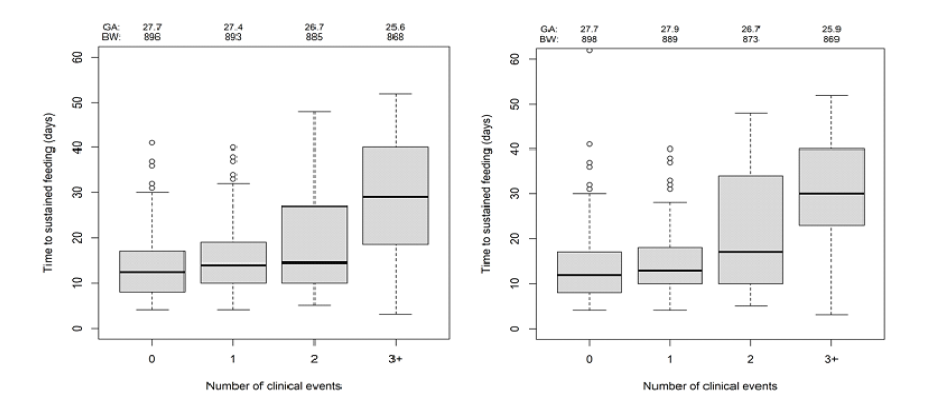
Figure 1.Time to SFT with use of the 10-day (A) and study-end definitions (B) for infants with adverse clinical outcomes in 0, 1, 2 or 3+ outcome categories as well as the average body weight and GA at birth. The box plots illustrate median values with 25th and 75th percentiles, extreme values within 1.5 times of the interquartile range (bars) and values even further away (o)
The study-end period for defining SFT was chosen by the sponsor, while the Expert group advised on a 10-day duration as more appropriate as this period would be less susceptible to spurious reductions in enteral feedings like elective surgery for persistent ductus arteriosus and inguinal hernia as well as retinal screenings etc. The quantitative statistical analysis demonstrated strong correlations to critical clinical outcomes (AEs) for a one-day change in the time to SFT. This poses a convincing argument for the clinical relevance of the evaluated primary endpoint parameter.
The 10-day definition of SFT showed stronger correlations to the one-day shift in SFT and allowed analysis of a higher number of infants. These infants also displayed a higher incidence of adverse clinical outcomes, which included the proportional incidences of NEC, gastrointestinal AEs as well as late onset sepsis and use of systemic antibiotics. As the body weight increase was the same for both groups, it could be argued that the 10-day SFT definition is more robust and more adequately mirrors requisites for maintaining a full enteral feeding in the infants. SFT-10 may be useful for future analyses of sustained feeding tolerances in the investigated patient cohort and potentially become a surrogate for adverse outcomes in premature infants.
Abbreviations
AEs: Adverse Events, BW: Birth Weight, CFU: Colony Forming Units, CTX: Clinical Trial Exemption, ELBW: Extremely low birth weight infants, cGA: corrected Gestational Age, g: grams, GMP: Good Manufacturing Practice, IND: Investigative New Drug, IQR: Interquartile Range, NEC: Necrotizing Enterocolitis, SAE: Serious Adverse Event, SFT: Sustained Feeding Tolerance, Sd: Standard deviation, UK: United Kingdom, US: United States
Acknowledgements
We are greatly indebted to all staff in the involved Neonatal Intensive Care Units as well as parents and relatives making this evaluation of premature infants possible.
Conflict of Interest Declaration
Dr Josef Neu is a consultant medical expert of Infant Bacterial Therapeutics and the global coordinating investigator of the ‘Connection Trial’ sponsored by Infant Bacterial Therapeutics.
Dr Teresa Del Moral is a principal investigator of the ‘Connection Trial’ sponsored by Infant Bacterial Therapeutics.
Dr Jenelle Ferry is a principal investigator of the ‘Connection Trial’ sponsored by Infant Bacterial Therapeutics
Dr Scott O Guthrie has received payments for consultancy in research from ONY Biotech and is a principal investigator of the ‘Connection Trial’ sponsored by Infant Bacterial Therapeutics.
Dr Andrea Nagy is a principal investigator of the ‘Connection Trial’ sponsored by Infant Bacterial Therapeutics
Dr Ajay Talati is a principal investigator of the ‘Connection Trial’ sponsored by Infant Bacterial Therapeutics
Marcus Thuresson is a consultant statistician of Infant Bacterial Therapeutics.
Anders Kronström, Staffan Strömberg and Jonas Rastad are all employees of Infant Bacterial Therapeutics.
References
1. Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. 2007; 85: 629S-34S.
2. Hay WW. Strategies for feeding the preterm infant. Neonatol. 2008; 94: 245-54.
3. Ong KK, Kennedy K, Castaneda-Gutierrez E, et al. Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatr. 2015; 104: 974-86.
4. Wiswanathan S, McNelis K, Super D, et al. Standardized slow enteral feeding protocol and the incidence of necrotizing enterocolitis in extremely low birth weight infants. JPEN J Parenter Enteral Nutr. 2015; 39: 644-54.
5. de Waard M, Li Y, Zhu Y, et al. Time to full enteral feeding for very low-birth-weight infants varies markedly among hospitals worldwide but may not be associated with incidence of necrotizing enterocolitis: The NEOMUNE- neonutrinet cohort study. JPEN J Parenter Enteral Nutr. 2019; 43: 658-67.
6. Hay WW. Optimizing nutrition of the preterm infant. Chin J Contemp Pediatr .2017; 19: 1-21.
7. Young L, Oddie SJ, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2022; 1: CD001970.
8. Berseth CL. Neonatal small intestine motility: motor responses to feeding in term and preterm infants. J Pediatr. 1990; 117: 777-82.
9. Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2017; 8: CD001241.
10. Aceti A, Gori D, Barone G, et al. Probiotics and time to achieve full enteral feeding in human milk-fed and formula-fed preterm infants: Systematic review and meta-analysis. Nutrients. 2016; 8: 471. doi 10.3390/nu8080471.
11. Kreissl A, Sauerzapf E, Repa A, et al. Starting enteral nutrition with preterm single donor milk instead of formula affects time to full enteral feeding in very low birthweight infants. Acta Paediatr. 2017; 106: 1460-7.
12. Dorling J, Hewer O, Hurd M, et al. Two speeds of increasing milk feeds for very preterm or very low-birthweight infants: the SIFT RCT. Health Technol Assess. 2020; 24: 1-94.
13. Wang N, Zhang J, Wang B, et al. Transition from parenteral to enteral nutrition and postnatal growth in very preterm infants during their first 28 days of life. Frontiers Pediatr. 2022; 10: 1-8.
14. Lewis ZT, Shani G, Masarwe CF, et al. Validating bifidobacterial species and subspecies identity in commercial probiotic products. Pediatr Res. 2016; 79: 445-52.
15. Juber BA, Boly TJ, Pitcher GJ, et al. Routine administration of a multispecies probiotic containing Bifidobacterium and Lactobacillus to very low birth weight infants had no significant impact on the incidence of necrotizing enterocolitis. Frontiers Pediatr. 2021; 9: 1-10.
16. Pointdexter B. Use of probiotics in preterm infants. Pediatrics. 2021; 147: e202151485.
Received: August 08, 2022;
Accepted: August 24, 2022;
Published: September 22, 2022.
To cite this article : Josef Neu , Teresa Del Moral, Jenelle Ferry, et al. Clinical Outcomes Correlating to a One-Day Shift in Sustained Feeding Tolerance in Very Low Birth Weight Infants in the ‘Connection Trial’. British Journal of Gastroenterology. 2022; 4(2): 255-260. doi: 10.31488/bjg.1000132.
© Josef Neu, et al. 2022.
