Review Article / Open Access
DOI: 10.31488/bjg.1000121
Complete Response of Metastatic Pancreatic Cancer In A CHEK2 Mutation Carrier Treated With Platinum-Based Therapy- A Case Report And Literature Review
Kai Sun1, Humaira Sarfraz1, Maen Abdelrahim*1,2
1.Houston Methodist Cancer Center, 6445 Main Street, Houston, Texas 77030, USA
2.Cockrell Center of Advanced Therapeutics Phase I Program, Houston Methodist Research Institute and Weill Cornell Medical College, Houston, TX 77030, USA
* Corresponding author: Maen Abdelrahim,Houston Methodist Cancer Center, 6445 Main Street, Houston, Texas 77030, USA
Abstract
A 53-year-old woman presented with abdominal pain and obstructive jaundice and was diagnosed with metastatic pancreatic adenocarcinoma. She received FOLFIRINOX chemotherapy and achieved complete response. Germline testing detected CHEK2 and treatment was switched to Olaparib maintenance.
Keywords: Pancreatic adenocarcinoma, CHEK2 mutation, BRCA mutation, PARPi, complete response
Introduction
Pancreatic adenocarcinoma is a major cause of cancer-related death [1]. The treatment and prognosis of patients with pancreatic cancer has made little progress over the past two decades compared to other solid tumor malignancies. Gemcitabine based chemotherapy had been the mainstream of treatment of metastatic pancreatic cancer. Gemcitabine with the combination of other agents have been explored with little improvement of overall survival. The combination of nab-paclitaxel and gemcitabine was one of the first combinations to show a significant improvement ofmedian overall survival to 8.5 months [2]. Later, more aggressive combination chemotherapy of irinotecan, oxaliplatin and fluorouracil further improved median overall survival. However, median overall survival is still less than one year [3].Majority of patients have partial response or stable disease with those chemotherapies. Complete response of advanced-stage or metastatic pancreatic cancer is rarely reported, ranging from 0-0.6% in major clinical trials [3-5].
Hereditary disorders from germline mutations account for about 10% pancreatic cancers, 3- 11% of which are familial pancreatic cancers from CHEK2 mutations [6-8].
Here we report a case of metastatic pancreatic cancer with germline CHEK2 mutation who achieved complete response with platinum-based therapy followed by poly (ADP ribose) polymerase inhibitor maintenance. The rare combination of a complete response and germline CHEK2 mutation prompted the question if CHEK2 mutation is an indicator of platinum sensitivity and the complete response was a result of this uncommon mutation. We then reviewed and summarized current literature. We alsohighlight the importance of understanding molecular profile and choosing therapy accordingly to achieve favorable outcomes.
Case presentation
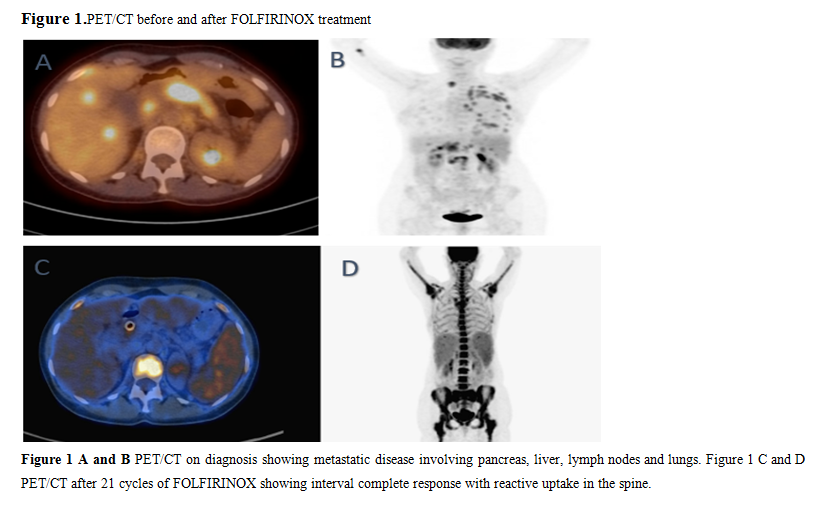
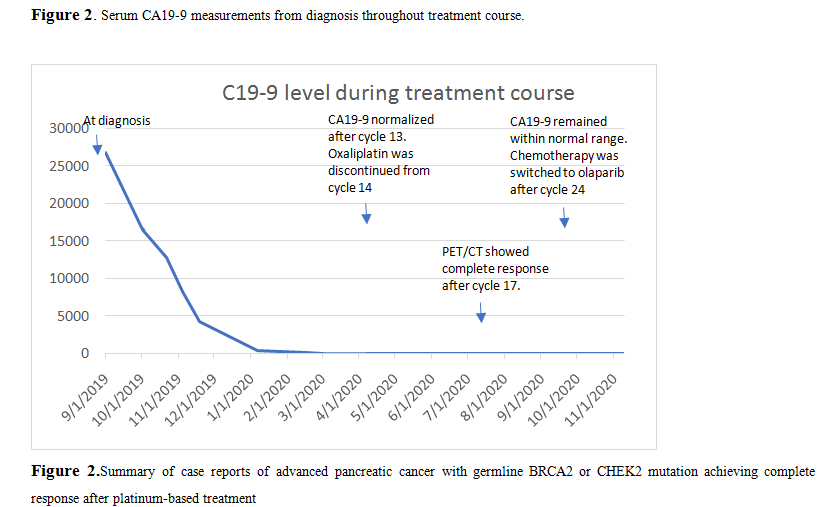
A 53-year-old woman with a past medical history of papillary thyroid cancer which was treated with surgery, presented to an outside hospital with obstructive jaundice, abdominal pain for 5 days and a 10 lbs weight loss in two weeks. A computerized tomography (CT) of abdomen and pelvis revealed a heterogeneous lesion at the isthmic portion of pancreas with evidence of arterial and venous invasion. A biliary stent was placed, and fine needle aspiration of the lesion was performed. Pathology is consistent with pancreatic adenocarcinoma. Molecular testing was performed and revealed KRAS, TP53 and SMAD4 mutations. Genetic testing revealed pathogenic heterozygous CHEK2 IVS2+ 1G>A mutation. A positron emission tomography (PET)/CT scan later confirmed the above findings and in addition showed intense uptake of retroperitoneal, mediastinal, left supraclavicular lymphadenopathy, hepatic, pulmonary, pleural and L1, L2 bone metastases (Figure 1 A and B). CA 19-9 was 26845 U/ml (range: 0-35 U/ml) upon diagnosis. She was started on mFOLFIRINOX (oxaliplatin 85 mg/m2, folinic acid 400 mg/m2, fluorouracil 2400 mg/m2 over 46 hours, irinotecan 150 mg/m2 every 14 days) chemotherapy. Restaging scan after cycle 8 of mFOLFIRINOX showed significant partial response. She continued to showresponse and oxaliplatin was discontinued after cycle 15 to minimize neurotoxicity. CA 19-9 continued to trend down and normalized after cycle 13 and remained within normal range (Figure 2). A PET/CT scan after cycle 21 showed complete response (Figure 1 C and D). Chemotherapy was switched to olaparib 300 mg twice a day maintenance after completion of 24 cycles of chemotherapy.
Discussion
CHEK2 is a kinase expressed throughout the cell cycle and activated mainly by ATM in response to double-strand DNA breaks. CHEK2 along with ATM, CHEK1, BRCA1, BRCA2 and other genes comprises of homologous recombination repair pathway that plays a major role in DNA damage repair, especially in double-strand DNA damage [9]. Four major pathogenic CHEK2 mutations have been described: c.1100delC, del5395bp, IVS2+ 1G>A and I175T. The deletion mutations lead to premature protein truncation at codon 381 and the splice site mutation (IVS2+ 1G>A) results in a 4-bp insertion and creates an aberrantly spliced CHEK2 mRNA encoding a truncated protein. I157T missense mutations leads to defective binding to BRCA1 and p53[10,11].Previous study[12], has shown that truncated CHEK2 proteins are associated with higher chance of thyroid cancer (odds ratio 4.9; P=0.0006), breast (OR=2.2; P=0.02) and prostrate (OR 2.2; P=0.04) while the missense mutation is associated more with breast cancer (OR 1.4; P=0.02), colon cancer OR 2.0; P=0.001), kidney cancer (OR 2.1; P=0.0006), prostate cancer (OR 1.7; P=0.002) and thyroid cancer (OR 1.9; P=0.04). All the thyroid cancers noted to be associated with CHEK2 mutation had papillary histology as in the case of our patient.
Due to the low frequency of CHEK2 mutation in pancreatic cancer, large studies of its significance and outcome including response to treatment are lacking. Two case reports [13,14], of metastatic pancreatic cancer with mutations of CHEK2 achieving complete remission after platinum-based chemotherapy were retrieved (Table 1). Interestingly, both cases had concurrent germline CHEK2 and BRCA mutations. Further literature search found several cases of pancreatic cancer with BRCA1 or BRCA2 mutations achieving complete response after platinum -based therapy [13,15,17]. Sonnenblick et al [15], reported a case of recurrent metastatic pancreatic adenocarcinoma after Whipple’s. The patient did not respond to gemcitabine treatment initially but achieved complete response after cisplatin was added. The case reported by Mathew et al [16], had an initial dramatic response with gemcitabine and oxaliplatin but developed resistance when oxaliplatin was discontinued, then had another long-lasting response when oxaliplatin was re-introduced later. Aguirre et al [13], reported a case series involving 6 patients with BRCA1 or BRCA2 mutations (5 germline and 1 somatic) and 5 out of 6 patients demonstrated long-lasting response to platinum treatment. In addition, 5 out of 8 patients with ATM, ATR or CHEK2 mutations treated with oxaliplatin-based chemotherapy showed clinical benefit.
Homologous recombination repair deficiency (HRD) leads to genome instability allowing cells to acquire multiple genetic mutations. It has been exploited as a target of DNA damaging agent- platinum, based on the hypothesis that cancer cells with HRD cannot repair DNA damage caused by platinum. This hypothesis has been proven in breast cancer and ovarian cancer[18,19]. Those cases of pancreatic cancer with CHEK2 and/or BRCA1/2 mutations achieving complete response after platinum-based therapy further supported this hypothesis in pancreatic cancer. In addition, a large retrospective study [20], showed that advanced-stage pancreatic adenocarcinoma with HRD had statistically significant improved overall survival and progression free survival when treated with first-line platinum-based chemotherapy.
| Age/gender | Mutation | Stage | Metastatic site | Treatment | Response | Duration of response (month) | |
|---|---|---|---|---|---|---|---|
| Our case | 53/F | CHEK2 IVS2+ 1G>A | Metastatic | Liver | FOLFIRINOX | CR | 14 |
| Pazderová et al | Unavailable | BRCA2, CHEK2 | Metastatic | Liver | FOLFIRINOX | CR | 30 |
| Aguirre et al | 58/F | CHEK2, BRCA1 | Metastatic | Liver | FOLFIRINOX followed by PARPi | Alive | 21 |
| Sonnenblick et al | 60/M | BRCA2 1153insT | pT3N1M0 recurred as metastatic disease | Liver | Gemcitabine with no response, cisplatin added for 8 months | CR | 10 |
| Gostimir et al | 61/F | BRCA2 E3002K | Borderline resectable | N/A | FOLFIRINOX | pCR | 15 |
| Mathew et al | 44/M | BRCA2 7990del3ins2 | Metastatic | Omentum | GEMOX+bev | CR | 24 |
| Aguirre et al | 63/F | BRCA1 | Metastatic | Liver | FOLFIRINOX->PARPi vs placebo trial | Alive | 5.7 |
| Aguire et al | 45/M | Somatic BRCA2 | Metastatic | Liver | FOLFIRINOX->PARPi | CR | 28 |
| Aguire et al | 64/M | BRCA2 | Metastatic | Liver | FOLFOX then Whipple | CR | 30 |
Abbreviations and units: M: Male; F: Female; FOLFIRINOX: oxaliplatin, folinic acid, fluorouracil and irinotecan; GEMOX: gemcitabine and oxaliplatin; Bev: bevacizumab; PARPi: poly (ADP ribose) polymerase inhibitor; CR: complete response; pCR: pathologic complete response; N/A: non-applicable.
Other therapeutic exploration of pancreatic cancer with HRD includes targeting compensatory repair pathway- poly (ADP-ribose) polymerase (PARP). PARP facilitates DNA repair by binding to DNA breaks and attracting DNA repair proteins to the site of damage[21]. Its activity is essential in cells with HRD. PARP inhibitors (PARPi) have shown both in vitro and in vivo activities in pancreatic cancer with BRCA mutations[21–23]. They have also shown effectiveness in ovarian cancer with HRD, especially in patients with a positive response to platinum-based therapy[24,25]. In the case series reported by Aguirre et al [13], several pancreatic cancer patients with CHEK2 and/or BRCA1/2 mutations had long-lasting response after platinum-based therapy was switched to PARPi maintenance.Clinical trial NCT02184195 is currently evaluating this approach of PAPRi as maintenance in BRCA mutated pancreatic cancer patients who responded to platinum-based therapy. Another trial (NCT01585805) is evaluating PARPi in combination with chemotherapy as first line treatment of metastatic pancreatic cancer with BRCA or PALB2 mutations. Given the above-mentioned evidence and a complete response our patient achieved with platinum-based therapy, she was started on olaparib maintenance.
In conclusion, we report a case of metastatic pancreatic cancer with germline CHEK2 mutation who achieved a complete response after platinum-based chemotherapy. Our report highlights the importance of testing pancreatic cancer patients of germline mutations and consider platinum-based therapy if CHEK2 mutation or other mutations involving homologous recombination pathway are detected. Maintenance PARPi can be an option however, more data is needed in controlled clinical trial setting.
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
CT: computerized tomography; BEV: bevacizumab; CR: complete response; F: Female; FOLFIRINOX: oxaliplatin, folinic acid, fluorouracil and irinotecan; GEMOX: gemcitabine and oxaliplatin; HRD: homologous recombination repair deficiency PET: positron emission tomography; PARPi: poly (ADP ribose) polymerase inhibitor; PCR: pathologic complete response; N/A: non-applicable; M: Male
References
1. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21.
2. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
3. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011 May 12;364(19):1817–25.
4. Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25(16):2212–7.
5. Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–52.
6. Chaffee KG, Oberg AL, McWilliams RR, et al. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet Med. 2018;20(1):119–27.
7. Bartsch DK, Krysewski K, Sina-Frey M, et al. Low frequency of CHEK2 mutations in familial pancreatic cancer. Fam Cancer. 2006;5(4):305–8.
8. Lener MR, Kashyap A, Kluźniak W, et al. The Prevalence of Founder Mutations among Individuals from Families with Familial Pancreatic Cancer Syndrome. Cancer Res Treat. 2017;49:(2):430–6.
9. Wright WD, Shah SS, Heyer W-D. Homologous recombination and the repair of DNA double-strand breaks. J Biol Chem. 2018; 29:3(27):10524–35.
10. Jalilvand M, Oloomi M, Najafipour R, et al. An association study between CHEK2 gene mutations and susceptibility to breast cancer. Comp Clin Path. 2017;26(4):837–45.
11. Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3(5):421–9.
12. Cybulski C, Górski B, Huzarski T, et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004;75(6):1131–5.
13. Aguirre AJ, Nowak JA, Camarda ND, et al. Real-time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer Discov. 2018;8(9):1096–111.
14. Pazderová N, Urbán V, Makovník M, et al. Complete Response to Chemotherapy in Metastatic Pancreatic Carcinoma Associated with Double Heterozygous Germline Mutation in BRCA2 and CHEK2 Genes - a Case Report. Klin Onkol. 2020;33(3):220–5.
15. Sonnenblick A, Kadouri L, Appelbaum L, et al. Complete remission, in BRCA2 mutation carrier with metastatic pancreatic adenocarcinoma, treated with cisplatin based therapy. Cancer Biol Ther. 2011 1;12(3):165–8.
16. Mathew BM, Daas AY, Centeno BA,et al. Lessons learned from a complete remission of advanced metastatic pancreatic ductal adenocarcinoma. Clin Adv Hematol Oncol. 2012 May;10(5):340–3.
17. Gostimir M, Bennett S, Moyana T, et al Complete pathological response following neoadjuvant FOLFIRINOX in borderline resectable pancreatic cancer - a case report and review. BMC Cancer. 2016 10;16(1):786.
18. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628–37.
19. Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014 ;20(3):764–75.
20. Park W, Chen J, Chou JF, et al. Genomic Methods Identify Homologous Recombination Deficiency in Pancreas Adenocarcinoma and Optimize Treatment Selection. Clin Cancer Res. 2020 ;26(13):3239–47.
21. Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7.
22. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50.
23. Andrei A-Z, Hall A, Smith AL, et al. Increased in vitro and in vivo sensitivity of BRCA2-associated pancreatic cancer to the poly(ADP-ribose) polymerase-1/2 inhibitor BMN 673. Cancer Lett. 2015 Aug 1;364(1):8–16.
24. González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019;381(25):2391–402.
25. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375(22):2154–64.
Received: July 08, 2020;
Accepted: : July 17, 2020;
Published: : : July 30, 2020.
To cite this article : Sun K, Humaira S, Maen A.Complete Response of Metastatic Pancreatic Cancer In A CHEK2 Mutation Carrier Treated With Platinum-Based Therapy- A Case Report And Literature Review. British Journal of Gastroenterology. 2020; 2:4..
©Maen A, et al.2020.
