Research Article / Open Access
DOI:10.31488/bjg.1000127
Effect of Bifidobacterium Bifidum Administration on Term Infants at 1 Year of Age: An Open-Label Randomized Control Trial
Satsuki Totsu1 , Ayako Nishiyama2,3, Masaki Terahara4 , Masaki Wada5 , Satoshi Kusuda6,7
1.Department of Neonatal Medicine, Sanno Hospital, Tokyo, Japan
2.Department of Pediatrics, Shiseikai Daini Hospital, Tokyo, Japan
3.Nishiyamakodomo Clinic. Tokyo, Japan
4.R&D Management Department, Meiji Co., Ltd., Tokyo, Japan
5.Department of Neonatology, Tokyo Women’s Medical University
6.Neonatal Research Network of Japan, Tokyo, Japan
7.Department of Pediatrics, Kyorin University, Tokyo, Japan
* Corresponding author: Satoshi Kusuda, Neonatal Research Network of Japan, Shinjuku Park Tower N30, 3-7-1, Shinjuku, Tokyo 163-1030, Japan
Abstract
Background: A single-center randomized controlled trial was conducted to explore the benefit of early life probiotic supplementation in term infants at one year of age. The CRT number was UMIN000029087. Methods: Term infants born between August 2017 and March 2018 were divided into the control group (CG) and intervention group (IG), which included infants born in the first and second half of the month, respectively. Infants in the IG received Bifidobacterium bifidum during the first month. The primary outcome was growth and development, and the secondary outcome was morbidity during the first year of life. The study was not masked; however, outcomes were assessed by a physician masked to the allocation. Differences in the primary outcome were analyzed using a t-test. Results: In total, 44 infants were enrolled in this study, 21 in the CG and 23 in the IG; however, nine infants were excluded during follow-up. Data of 17 infants in the CG and 18 in the IG were analyzed. Mean and standard deviation of body weight, body length, and head circumference of both groups at one year of age were 8.88 ± 0.74 vs. 9.13 ± 0.91 kg, 73.0 ± 1.9 vs. 73.7 ± 2.3 cm, and 45.7 ± 1.6 vs. 46.0 ± 1.1 cm, respectively. The developmental quotient was 111 ± 15 vs. 109 ± 14. There were no significant differences in the primary outcomes. However, in the secondary analysis, infants in the IG had significantly fewer days without defecation (9.9 vs. 16.3%, p < 0.01, chi-square test). Conclusions: Early life probiotic supplementation in term infants did not show beneficial effects on growth and development in the first year of life. However, bowel movements during the first year of life might be improved by probiotic supplementation.
Keywords: Bifidobacterium, growth, development, defecation, infant
Introduction
Bifidobacterium is the predominant genus in the intestinal tract of infants [1-3]. The benefit of Bifidobacterium administration in infants has been reported to have positive effects not only on the intestinal function and the immune system but also on growth and neurodevelopment [4,5]. The benefits are mainly expected in high-risk infants, such as preterm or low-birth-weight infants, who are at risk of impaired growth and poor neurodevelopment in infancy. However, the effects on healthy term infants, who have a low probability of suffering from physical and neurological developmental abnormalities, are still not well understood.
In preterm infants, colonization of the gut microbiota might be easily disrupted due to the effects of cesarean delivery, frequent antibiotic use, and has undergone several procedures in the neonatal intensive care unit (NICU) [6]. Therefore, the use of Bifidobacterium in these infants could aid in the colonization of microbes in the gut and prevent several morbidities, including those developed during the NICU stay, necrotizing enterocolitis (NEC), late-onset sepsis, and health problems in later life, such as asthma and eczema [7]. We previously reported on enhanced enteral feeding and reduced frequency of late-onset sepsis in very-low-birth-weight infants who received Bifidobacterium [8,9]. In addition, their neurodevelopment at 1.5 years of age also improved significantly [10].
Therefore, we conducted an exploratory clinical study to investigate the benefits of Bifidobacterium administration in term infants. The hypothesis of the study was that Bifidobacterium administration in healthy term infants would produce benefits as seen in high-risk infants.
Methods
Subjects
Healthy term infants who were born at Shiseikai Daini Hospital between August 2017 and March 2018 were included in this study. Infants diagnosed with serious infections or severe birth defects were excluded. Infants with other issues, such as multiple births, birth asphyxia, and respiratory instability as assessed by the attending physician, were also excluded.
The Internal Review Board of Siseikai Daini Hospital approved this study. Written informed consent was obtained from the parents of all infants enrolled in this study. Additionally, details that might disclose the identity of the infants under study were omitted in the manuscript to preserve anonymity.
Study design
The study design was a prospective open-label quasi-randomized controlled trial. The clinical trial registration number was UMIN000029087. The infants enrolled in this study were divided into control (CG) and intervention (IG) groups, in which Bifidobacterium bifidum OLB6378 strain was administered up to 1 month after birth according to the date of birth. Infants born in the first half of the month were allocated to the IG and those born in the second half to the CG. The subjects were followed up to 1 year of age. Although the study was not masked, the outcomes were assessed by a physician masked to the allocation of infants.
Intervention
Bifidobacterium bifidum OLB6378 sachets (0.5 g) containing approximately 5 × 109 cfu bacteria were provided by Meiji Co., Ltd. The protocol for Bifidobacterium administration was similar to that followed in our previous study involving preterm infants [9]. Bifidobacterium was administered to term infants for up to 1 month after birth. Infants in the IG received Bifidobacterium twice a day, starting within five days after birth. The contents of the sachet were dissolved in 2 mL of distilled water, and 1 mL of the solution was orally administered to the infant with a dropper. Once a total of 60 sachets were completed, Bifidobacterium administration was discontinued. Infants in the CG did not receive any intervention.
Basic and follow-up data
Basic information, including birth date, gestational age, birth weight, length and head circumference at birth, sex, delivery method, Apgar scores, feeding method, number of siblings, and family history of allergies was collected.
Data about morbidities, including hospital visits, cough, nasal discharge, diarrhea, and fever, were collected until the infants turned one year old. Allergic diseases, including atopic dermatitis, asthma, and food allergies, were diagnosed by the same physician who followed up on the infants. In addition, daily bowel movements were recorded by the parents. Details of daycare use, vaccination history, and weaning diet were also recorded. A medical checkup was conducted at one year of age, which included body weight, height, and head circumference measurements and the developmental quotient (DQ) assessed by the Enjoji-style infant development test [11].
Endpoints
The primary endpoints were set as follows:
(1) Improvement in body indices (height, weight, head circumference, BMI)
(2) Improvement in the neurodevelopmental index
The secondary endpoint was set as morbidities diagnosed and bowel movement up to 1 year of age.
Sample size
Since this was an exploratory study, only the recruitment period of 8 months (August 2017 to March 2018) was set in advance. Based on the expected number of births during this study period, which we calculated to be around 300, almost 100 infants (50 infants in each group) were expected to be enrolled in the study, which would have been adequate to observe a 20% improvement in growth and neurodevelopment in the IG.
Statistical analyses
All results were analyzed using IBM SPSS Statistics version 27.0 (IBM Corp., Armonk, NY, USA). Continuous variables were tested using the t-test or U-test and categorical variables using the chi-square test, with a significant difference set at p < 0.05.
Results
Study population
During the 8-month study period, 220 infants were born in the hospital, 44 of whom were enrolled after obtaining consent from their parents. Based on the birth date randomization, 21 and 23 infants were allocated to the CG and IG, respectively. Enrollment was stopped at the end of the study period. Due to the unexpectedly low number of deliveries due to a shortage in obstetrical staff and the low consent rate for the study, the number of infants enrolled was much below our expectations. Therefore, the sample size was predetermined as one-fourth of the expected sample.
Four infants in the CG and five infants in the IG were lost to follow-up and excluded from the study. Therefore, the final analysis was performed using the data on 17 infants in the CG and 18 in the IG (Figure 1).
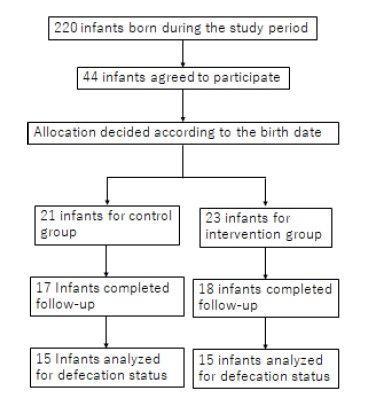
Figure 1.Study Population
For the secondary analysis of bowel movements, 15 infants from each group were examined.
Background characteristics
The background characteristics of the enrolled infants are presented in Tables 1 and 2. There was a significantly higher proportion of boys in the IG than in CG (p < 0.05, chi-square test). However, there was no difference between groups in vaginal delivery rate, gestational age, birth weight, Apgar scores at 1 and 5 minutes, breastfeeding rate at discharge from the hospital, family history of allergic diseases, presence of pet animals in the home, and presence of smokers in the home. The number of doses of Bifidobacterium bifidum in the IG was 55.2 ± 5.7, indicating an intake rate of more than 90%.
Table 1.Background characteristics
| Group | Control Group (n=17) | Intervention Group (n=18) | p |
|---|---|---|---|
| Vaginal delivery (%) | 76.5(13/17) | 81.3(13/16) | ND*1 |
| Boy/Girl | 5/12 | 12/6 | <0.05*1 |
| Gestational Age (week) | 39.0±0.8 | 38.8±0.9 | ND*2 |
| Birth weight (g) | 3004±205 | 3028±343 | ND*2 |
| Apgar score at 1 min | 8.1±0.2 | 8.1±0.3 | ND*2 |
| Apgar score at 5 min | 8.8±0.4 | 8.8±0.4 | ND*2 |
| Breast feeding (%) | 70.6(12/17) | 77.8 (14/18) | ND*1 |
*1: chi-square test; *2: t-test; ND: no significant difference
Table 2.Background characteristics 2
| Group | Control Group (n=17) | Intervention Group (n=18) | p |
|---|---|---|---|
| Family history of allergic diseases (%) | 100(17/17) | 77.8(14/18) | ND*1 |
| Pet animals (%) | 17.6(3/17) | 5.6(1/18) | ND*1 |
| Smoker at home (%) | 52.9(9/17) | 27.8(5/18) | ND*1 |
*1: chi-square test; ND: no significant difference
Primary outcomes
Table 3 shows the mean and standard deviation (SD) of body weight, body length, head circumference, and DQ for both groups. There were no statistically significant differences in the primary outcomes between the groups.
Table 3.Growth and development at 1 year of age
| Group | Control Group (n=17) | Intervention Group (n=18) | p |
|---|---|---|---|
| Body weight (kg) | 8.88±0.74 | 9.13±0.91 | ND*1 |
| Body length (cm) | 73.0±1.9 | 73.7±2.4 | ND*1 |
| Head circumference (cm) | 45.7±1.6 | 46.0±1.1 | ND*1 |
| DQ | 111±151 | 109±14 | ND*1 |
*1: t-test; ND: no significant difference; DQ: developmental quotient
Secondary outcomes
Table 4 shows the number of hospital visits and the number of days with symptoms indicative of a common cold, which were not significantly different between the groups. There was also no difference in the incidence of allergic diseases. Table 5 shows bowel movements. The number of days without defecation over the total observed days was significantly lower in the IG (p < 0.01, chi-square test). Furthermore, the average duration without defecation was significantly lower in the IG. However, the incidence of constipation did not show a significant difference due to the small number of infants with constipation based on the clinical guideline definition [12].
Table 4.Number of hospital visits, and days and episode of cold symptoms
| Group | Control Group (n=17) | Intervention Group (n=18) | p |
|---|---|---|---|
| Number of hospital visits | 9.7±9.1 | 8.4±6.3 | ND*1 |
| Days of symptoms | |||
| Cough | 24.8±21.6 | 22.3±25.2 | ND*2 |
| Nasal discharge | 38.5±42.4 | 31.3±20.0 | ND*2 |
| Fever | 6.9±4.8 | 8.8±7.8 | ND*2 |
| Episodes of fever | 3 .7±2.6 | 4.1±3.5 | ND*1 |
| Allergic diseases | ND*3 | ||
| Atopic dermatitis | 2 | 2 | |
| Asthma | 2 | 0 | |
| Food allergy | 3 | 4 |
*1: chi-square test; *2: Mann-Whitney U-test; ND: no significant difference; Numbers of days in parenthesis.
There was no significant difference in the number of days with diarrhea between the groups. There was no significant difference in the number of months after which the weaning diet was initiated, 5.4 ± 0.5 vs. 5.4 ± 0.7 months in the CG and IG, respectively.
Sensitivity analysis
The effects of Bifidobacterium administration may be diminished in breastfed infants. Therefore, although the number of cases decreased, bowel movements were analyzed in the same manner only for breastfed infants at hospital discharge. The number of cases was 10 in the CG and 12 in the IG. The results of the analysis are shown in Table 6. Although some of the significant differences observed in all cases disappeared, no change in the tendency of improved bowel movements was observed in infants administered Bifidobacterium as a whole.
Table 5.Bowel movement
| Group | Control Group (n=15) | Intervention Group (n=15) | p |
|---|---|---|---|
| Rate of days without defecation (%) | 16.2(683/4199) | 9.9(385/3871) | <0.01*1 |
| 1 day (%) | 6.7(280) | 5.7(221) | ND*1 |
| 2 days (%) | 4.0(166) | 2.6(102) | <0.01*1 |
| 3 days (%) | 2.4(102) | 0.9(36) | <0.01*1 |
| 4 days (%) | 1.2(52) | 0.4(16) | <0.01*1 |
| >4 days (%) | 2.0(83) | 0.3(10) | <0.01*1 |
| Average duration without defecation (day) | 1.5±0.5 | 1.1±0.5 | <0.05*2 |
| Number of infants who defecated <3 times a week more than 1 month | 3 | 0 | ND*1 |
| a week for >1 month |
*1: chi-square test, *2: Mann-Whitney U-test; ND: no significant difference; Numbers of days in parenthesis.
Table 6.Bowel movement among the infants who were breast fed at hospital discharge (Sensitivity analysis)
| Group | Control Group (n=10) | Intervention Group (n=12) | p |
|---|---|---|---|
| Rate of days without defecation (%) | 17.6(473/2695) | 9.9(276/2776) | <0.01*1 |
| 1 day (%) | 6 . 4 ( 1 7 2 ) | 5.1(142) | ND*1 |
| 2 days (%) | 3.9(104) | 2.8(78) | ND*1 |
| 3 days (%) | 2 . 8 ( 7 5 ) | 1.1(30) | <0.01*1 |
| 4 days (%) | 1 . 5 ( 4 0 ) | 0.1(16) | <0.01*1 |
| >4 days (%) | 3 . 1 ( 8 3 ) | 0 . 4 ( 1 0 ) | <0.01*1 |
| Average duration without defecation (day) | 1.5±0.5 | 1.1±0.5 | ND*1 |
| Number of infants who defecated <3 times a week more than 1 month | 2 | 0 | ND*1 |
| a week for >1 month |
*1: chi-square test; *2: Mann-Whitney U-test; ND: no significant difference; Numbers of days in parenthesis.
Discussion
In this study, Bifidobacterium administration to healthy term infants did not show any beneficial effects on growth and neurodevelopment in the first year of life, the primary endpoints. However, the secondary endpoint, the number of days without defecation, was significantly lower in infants who received Bifidobacterium. This benefit was also shown as a significant reduction in duration without defecation. Other secondary endpoints did not show any significant benefits or adverse outcomes between the groups.
Bifidobacterium was administered only from the early postnatal period to one month after birth, but the improvement in intestinal function was observed even up to one year after birth. In adults, the administration of prebiotics and synbiotics has shown improvements in gut microbiota and constipation [13,14]. Similarly, the administration of prebiotics in infants has been reported to improve bacterial flora and bowel movements, although these reports are limited [15–17]. Therefore, our study, in which Bifidobacterium was administered to term infants, makes a valuable contribution to the literature. It must be noted that this was only an exploratory study, and the intestinal bacterial flora of infants was not examined. Further studies are required to verify the benefits of Bifidobacterium administration in infants by using a larger sample and examining the intestinal flora.
Enhanced growth and neurodevelopment following the administration of Bifidobacterium, which was the primary endpoints of this study, were not observed. These results were not consistent with those of our previous studies involving very-low-birth-weight infants, which showed improved enteral nutrition and neurodevelopment following Bifidobacterium administration [10]. This discrepancy could be explained by the difference in the background risk of developmental delay between high-risk and healthy term infants. In high-risk infants, developmental delay can be mitigated by Bifidobacterium administration. However, term infants do not have a risk of delayed nutrition or delayed neurodevelopment, hence the use of Bifidobacterium might not have contributed much to stimulating their growth or improving their neurodevelopment. Further studies are necessary to validate these findings [18].
No significant differences in the secondary endpoints except bowel movement were observed between the two groups. In particular, there were no differences in the prevalence of upper respiratory tract infections. Infants delivered by cesarean section were reported to have intestinal bacterial flora that showed poorer Bifidobacterium colonization compared to infants born vaginally [19,20]. Furthermore, it is speculated that a disturbed intestinal flora might increase children’s susceptibility to upper respiratory tract inflammation [21]. In this study, approximately 40% of infants in the IG and 30% in the CG were born by cesarean delivery; this difference in numbers is likely why we observed similar outcomes in both groups. The incidence of upper respiratory tract infection also did not differ between the groups. This result could not be confirmed due to either the small sample size or the lack of efficacy of the probiotic in term infants.
This exploratory study has some limitations. First, the groups were not masked. To overcome this, the evaluation of infants was performed by physicians masked to their allocation, thus avoiding observer bias. Second, the bacterial flora in the feces was not examined. Therefore, it might not be possible that improved bowel movements can directly indicate improved bacterial flora in the IG. Third, it is known that intestinal flora is improved by breastfeeding [22]. If the number of infants in the study was large enough to divide into subgroups for analysis, such as vaginal delivery vs. cesarean section, breastfeeding vs. formula feeding, etc., the study might have shown a more clear benefit of the use of probiotics. However, the sensitivity analysis supported the beneficial effect on bowel movements even among the breastfed infants at hospital discharge only.
Even though Bifidobacterium administration in term infants lasted only up to one month after birth, the improvement in bowel movements continued until the infants were one year of age. A larger-scale trial with masking could only verify this benefit. Currently, a randomized double-blinded clinical trial with a larger sample size is being conducted by our group.
Conclusion
Although this was an exploratory study with a small sample, it is worth noting that it demonstrated an improvement in bowel movements in infants administered Bifidobacterium. With further studies, we may be able to recommend giving probiotics to term infants suffering from constipation.
Abbreviations
NICU: Neonatal Intensive Care Unit; NEC: Necrotizing Enterocolitis; CG: Control Group; IG: Intervention group; DQ: Developmental Quotient; SD: Standard Deviation
Acknowledgments
We thank all the families who participated in this trial, as well as all the staff at Shiseikai Daini Hospital.
Authorship Contributions
Satsuki Totsu had a major role in study design and data analysis and drafted the initial manuscript, and takes final responsibility for the decision to submit for publication. Ayako Nishijima was involved in study design, enrolment of infants, and running the trial and approved the final manuscript as submitted. Masaki Terahara was involved in running the trial and reviewed the manuscript, and approved the final manuscript as submitted. Masaki Wada was involved in study design and data analysis and approved the final manuscript as submitted. Satoshi Kusuda, the corresponding author, had a major role in study design, data collection, and interpretation of results and approved the final manuscript as submitted.
Disclosure Statement
This study was partially funded by the Food Science Institute Foundation, Japan. This funder had no role in the study design, data collection, data analysis, data interpretation, manuscript writing, or decision to publish. The interventions for this trial were provided by Meiji Co., Ltd (Tokyo, Japan). Masaki Terahara is employed by Meiji Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Mitsuoka T. Development of functional foods. Biosci. Microbiota Food Health. 2014;33(3):117-28. Available from: doi: 10.12938/bmfh.33.117.
2. Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690-703. Available from: doi: 10.1016/j.chom.2015.04.004.
3. Nagpal R, Kurakawa T, Tsuji H, et al. Evolution of gut Bifidobacterium population in healthy Japanese infants over the first three years of life: a quantitative assessment. Sci. Rep. 2017;7(1):10097. Available from: doi: 10.1038/s41598-017-10711-5.
4. Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017;66(4):515-22. Available from: doi: 10.1016/j.alit.2017.07.010
5. Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010;10(3):159-69. Available from: doi: 10.1038/nri2710.
6. Walker WA. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr. Res. 2017;82(3):387-95. Available from: doi: 10.1038/pr.2017.111.
7. Millar M, Seale J, Greenland M, et al. The Microbiome of Infants Recruited to a Randomised Placebo-controlled Probiotic Trial (PiPS Trial). EBioMedicine. 2017;20:255-62. Available from: doi: 10.1016/j.ebiom.2017.05.019
8. Yamasaki C, Totsu S, Uchiyama A, et al. Effect of Bifidobacterium administration on very-low-birthweight infants. Pediatr. Int. 2012;54(5):651-6. Available from: doi: 10.1111/j.1442-200X.2012.03649.x.
9. Totsu S, Yamasaki C, Terahara M, et al. Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial. Pediatr. Int. 2014;56:714-9. Available from: doi: 10.1111/ped.12330
10. Totsu S, Terahara M, Kusuda S. Probiotics and the development of very-low-birth-weight infants: Follow up study of a randomized trial. BMJ Paediatr. Open. 2018;2(1):e000256. Available from: http://dx.doi.org/10.1136/bmjpo-2018-000256
11. Enjoji M, Aiya N: Enjoji Scales of Infant Analytical Development Test. Tokyo, 1980, Keioutusin (in Japanese)
12. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393-1407. Available from: doi: 10.1053/j.gastro.2016.02.031.
13. Yu T, Zheng Y, Tan JC, et al. Effects of prebiotics and synbiotics on functional constipation. Am. J. Med. Sci. 2017;353(3):282-92. Available from: doi: 10.1016/j.amjms.2016.09.014
14. Fuyuki A, Higurashi T, Kessoku T, et al. Efficacy of Bifidobacterium bifidum G9-1 in improving quality of life in patients with chronic constipation: a prospective intervention study. Biosci. Microbiota Food Health. 2021;40(2):105-114. Available from: doi: 10.12938/bmfh.2020-073
15.. Alcon-Giner C, Dalby MJ, Caim S, et al. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: an observational study. Cell Rep. Med. 2020;(5)1:100077. Available from: doi: 10.1016/j.xcrm.2020.100077
16. Martín R, Bottacini F, Egan M, et al. The infant-derived Bifidobacterium bifidum strain CNCM I-4319 strengthens gut functionality. Microorganisms. 2020;8(9):1313. Available from: doi: 10.3390/microorganisms8091313
17. Navarro-Tapia E, Sebastiani G, Sailer S, et al. Probiotic supplementation during the perinatal and infant period: effects on gut dysbiosis and disease. Nutrients. 2020;12:2243. Available from: doi: 10.3390/nu12082243
18. Cohen Kadosh K, Muhardi L, Parikh P, et al. Nutritional support of neurodevelopment and cognitive function in infants and young children-an update and novel insights. Nutrients. 2021;13(1):199. Available from: doi: 10.3390/nu13010199
19. Nagpal R, Yamashiro Y. Gut Microbiota composition in healthy Japanese infants and young adults born by c-section. Ann. Nutr. Metab. 2018;73(Suppl 3):4-11. Available from: https://doi.org/10.1159/000490841
20. Hoang DM, Levy EI, Vandenplas Y. The impact of Caesarean section on the infant gut microbiome. Acta Paediatr. 2021;110:60-7. Available from: doi: 10.1111/apa.15501
21. Li KL, Wang BZ, Li ZP, et al. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J Pediatr. 2019;15:255-261. Available from: doi: 10.1007/s12519-019-00248-0.
22. Hurkala J, Lauterbach R, Radziszewska R, et al. Effect of a short-time probiotic supplementation on the abundance of the main constituents of the gut microbiota of term newborns delivered by cesarean section-a randomized, prospective, controlled clinical trial. Nutrients. 2020;12(10):3128. Available from: doi: 10.3390/nu12103128
Received: March 02, 2022;
Accepted: March 18, 2022;
Published: March 20, 2022.
To cite this article : Totsu S, Nishiyama A, Terahara M. Effect of Bifidobacterium bifidum Administration on Term Infants at 1 Year of Age: An Open-Label Randomized Control Trial. British Journal of Gastroenterology. 2022; 4(1): 229- 234. doi: 10.31488/bjg.1000127.
© Totsu S, et al. 2022.
