Research Article / Open Access
DOI:10.31488/bjg.1000125
Effects and Clinical Significances of Orally Supplementary Soybean β-Conglycinin in Subjects with Dyslipidemia and Renal Dysfunction
Fukuhiro Ueda1, Kentaro Ohtoshi2, Mitsutaka Kohno3, Kyoko Taku4, Yutaka Harano5
1. Department of Food and Nutrition, Hagoromo University of International Studies, Osaka, Japan
2. Ohtoshi Internal Medical Clinic, Osaka, Japan
3. Sports Nutrition and Health Center, Kyusyu Kyoritsu University, Fukuoka, Japan
4. Research Institute for Creating the Future, Fuji Oil Holdings Inc. Ibaraki, Japan
5. Niseikai Center of Lifestyle-Related Diseases, Harano Clinic, Osaka, Japan
* Corresponding author: Yutaka Harano MD,PhD, Niseikai Center for Lifestyle-Related Diseases, Harano Clinic, Osaka, Japan, Tel: +81-72-700-1135; Fax: +81-72-700-1135
Abstract
Soybean β-conglycinin, which is a main component of soy protein, is known to be the active ingredient which is thought to be effective as a supplement to correct dyslipidemia and related over weight. A large number of patients with impaired renal function also have dyslipidemia. The combined use of statin and fibrate in subjects with renal dysfunction is not recommended. This study was designed to determine the supplementary usefulness of soybean β-CG to manage dyslipidemia in subjects with renal dysfunction. The study used non-blind open study for 3 months. Blood samples were collected from each subject at the start and the end of the supplement intake. After the study period (for 3M), some subjects continued to take β-CG without restriction of protein intake and the beneficial effects of β-CG on the subsequent lipid metabolism and renal function were observed. The serum TG and LDL-C levels were significantly reduced after use of β-CG supplement. Functionally significant improvement was observed judging from the decrease of creatine levels. In the follow-up observation without reducing protein intake corresponding to the added supplement after the study period, the above effects persisted. β-CG is an effective supplementary food that will be useful for correcting dyslipidemia in subjects with impaired renal function.
Keywords: Renal dysfunction, dyslipidemia, TG, LDL-C, Soybean β-conglycinin :
Introduction
Chronic kidney disease often accompanies dyslipidemia. Statin is known as the most efficient agent for reducing plasma LDL-C. Fibrate is used in high TG and/or also used in combined hyperlipidemia (high TG and LDL-C). However, combinational use of statin and fibrate for patients with dyslipidemia having renal dysfunction are contraindicated.
β-CG, a major component of soy protein, has been reported to decrease serum TG, LDL levels and visceral fat [1-4]. In addition, β-conglycinin inhibits hepatic fatty acid synthesis, and improves insulin sensitivity [5-7]. Accordingly, β-conglycinin may help to improve lipid abnormalities in patients with renal dysfunction as a supplementary food.
The present study was performed to evaluate the efficacy of soybean β-conglycinin on hyperlipidemia (serum triglyceride ≥ 1.69 mmol/L and/or serum LDL-cholesterol ≥ 3.62 mmol/L) in subjects with renal dysfunction (Cr ≥ 88.4 μmol/L or eGFR < 60 ml/min/1.73m2) under carefully controlled conditioning of protein intake. In an additional study, the effects of β-CG intake on some subjects without restriction of the 5g protein intake have been observed for another 6 months after the study.
Materials and Methods
Supplementary food
Soybean β-conglycinin was manufactured by the method developed previously [8], and orally used in the form of candy type supplement. The powder of β-conglycinin consisted of 88% pure β-conglycinin, 5% other soy protein, 2% mineral and 5% water, which were sterilized for food use and spray dried from the defatted soy flowers. A nutritional composition of the supplement was shown in Table 1. The test candy contained 0.575 g of the pure β-conglycinin per piece (Table 1).
Table 1.Nutritional composition of the test candies
| Energy (kcal) | 46.0 |
| Protein (g) | 4.90 |
| Carbohydrate (g) | 6.56 |
| Lipid (g) | Trace |
These values indicate the amount of 8 pieces of candies consumed in a day.
Table 2.Clinical characteristics and serum levels of blood chemistries
| Unit | Standard value | Mean SE | |
|---|---|---|---|
| Number of subjects (male/female) | 14 (9/5) | ||
| Age | year | 73.6 ± 0.8 | |
| Height | cm | 160.3 ± 0.6 | |
| weight | kg | 64.9 ± 0.7 | |
| BMI | 25.3 ± 0.3 | ||
| SBP | mmHg | <135 | 151 ± 1.6 |
| DBP | mmHg | <85 | 80 ± 0.5 |
| TG | mmol/L | 0.56-1.68 | 2.45 ± 0.08 |
| LDL-C | mmol/L | 1.81-3.62 | 2.9 ± 0.04 |
| HDL-C | mmol/L | 1.03-2.33/1.03-2.66* | 1.07 ± 0.02 |
| FBG | mmol/L | 4.05-6.05 | 8 ± 0.24 |
| HbA1c | % | 4.9-6.0 | 6.4 ± 0.3 |
| Cr | μmol/L | 57.5-94.6/40.7-69.8* | 97.8 ± 2.8 |
| eGFR | ml/min/1.73m2 | ≥60 | 46 ± 0.8 |
| BUN | mmol/L | 2.86-7.14 | 8 ± 0.2 |
| AST | IU/L | 13-30 | 26.7 ± 2 |
| ALT | IU/L | 10-42/7-23* | 26.2 ± 3 |
| γ-GTP | IU/L | 13-64/9-32* | 28.4 ± 3.9 |
* Left, standard value for male; right, that for female
Subjects
All subjects (n=14) were those who complicated of renal dysfunction (Cr ≥ 88.4 µmol/L or eGFR < 60 ml/min/1.73m2) with dyslipidemia (serum TG ≥ 1.69 mmol/L and/or serum LDL-C ≥ 3.62 mmol/L) and who did not normalize with the standard diet treatment. Table 2 depicts the clinical characteristics and laboratory data of these subjects. The subjects have been clinically followed at the Center of Lifestyle-Related Diseases and Ohtoshi Internal Medical Clinic.
Study deigns
The study used non-blind open study for 3 months. Subjects were instructed to consume four pieces of candy twice a day containing a total of 4.6 g soybean β-conglycinin (Table 1), before breakfast and dinner. At the start of the study, patients were instructed to reduce protein intake (ca, 5g) in ordinary meals corresponding to the β-conglycinin proteins. After the study period (for three months), some subjects continued to intake β-conglycinin candies without restricting protein intake.
Ethical considerations
“Clinical usefulness & significance of isolated soybean protein for the management of obesity and dyslipidemia “study was approved by the ethics committee of Saiseikai Senri Hospital on Aug 19, 2015, and β-conglycinin use was included in this approved study. This study was also, conducted according to the guidelines of the Declaration of Helsinki, and written informed consent was obtained from all the participants.
Analyses of blood chemistry
Blood samples were taken from each subject at the start and the end of the study. Fasting blood samples were obtained in the morning before the breakfast. The hematological and serum biochemical measurements were carried out by SRL, Inc. (Tokyo, Japan) :the blood cell count, hemoglobin; TG, high-density lipoprotein-cholesterol (HDL-C), and LDL-C; aspartic acid aminotransferase (AST), alanine aminotransferase(ALT), gamma-glutamyl transpeptidase (γ-GTP); total protein, Cr, blood urea nitrogen (BUN), uric acid (UA), urine albumin and urine protein; plasma glucose (FPG) and HbAlc ; and Na, K, and Cl. eGFR was calculated from sex, age and Cr of each subject.
Statistical analysis
The paired t-test was used for comparisons of the initial and final clinical data. SPSS (SPSS 10.0J for Windows; SPSS Co., Tokyo, Japan) was used for all statistical analyses and a 5% significance level was applied in the two-tailed tests.
Results
Subjects
The subjects were mostly having type 2 diabetes, impaired glucose tolerance (IGT) or hypertension at G3a level from their mean eGFR values (46.0 ± 0.8 ml/min/1.73 m2, Table 2) in severity classification of chronic kidney disease (CKD) according to the guidebook of Japanese Society of Nephrology [9]. The average BMI of the subjects exceeded 25 (25.3 ± 0.3), and they corresponded to the initial or mild obesity according the guideline of the Japan Society for the Study of Obesity [10]. In addition, the subject's mean serum TG, blood pressure (systolic blood pressure, SBP), and FPG corresponded to dyslipidemia, hypertension or type 2 diabetes in their diagnostic guidelines [11-13].
Serum Lipid, Glucose, HbA1c and others
The mean serum TG was significantly reduced from 2.45 ± 0.08 mmol/L to 1.63 ± 0.03 mmol/L after 3 months of β-conglycinin intake (p=0.014). The mean serum LDL-C decreased to 2.54 ± 0.07 mmol/L from 2.90 ± 0.04 mmol/L significantly (p=0.029). The mean serum HDL-C did not change (Figure 1). No significant change was observed for FPG and HbA1c.
Urinary albumin, serum creatinine and e-GFR (Figure 2)
Urinary albumin/Cr ratio significantly reduced by 40% after 3m of supplementary treatment. The mean serum creatinine concentration reduced from 97.8 ± 2.8 μmol/L to 93.1 ± 2.2 μmol/L after 3 months of β-conglycinin intake with significant tendency (p=0.068). The mean eGFR, which was calculated from creatinine value, was significantly increased to 50.4 ± 3.7 ml/min/1.73 m2 from 46.0 ± 0.8 ml/min/1.73 m2 after 3 months of β-conglycinin consumption (p=0.040).
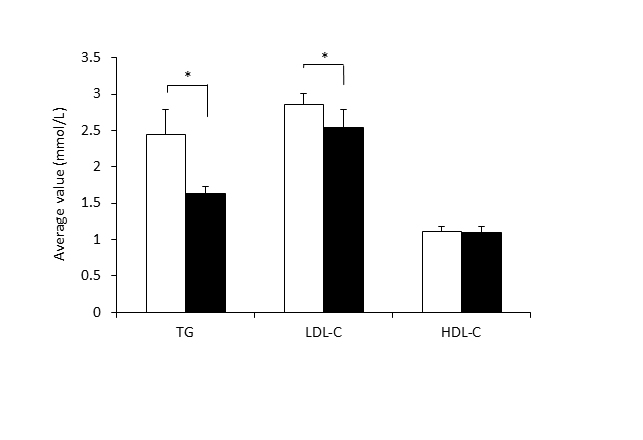
Figure 1.Changes of serum lipid levels. The results are expressed as means ± SE. (n=14). TG, triglyceride; LDL-C, LDL-cholesterol; HDL-C, HDL-Cholesterol.
White bar, Initial value; black bar, value after test period (3 months).
*significantly different from initial value (p < 0.05) by paired t-test.
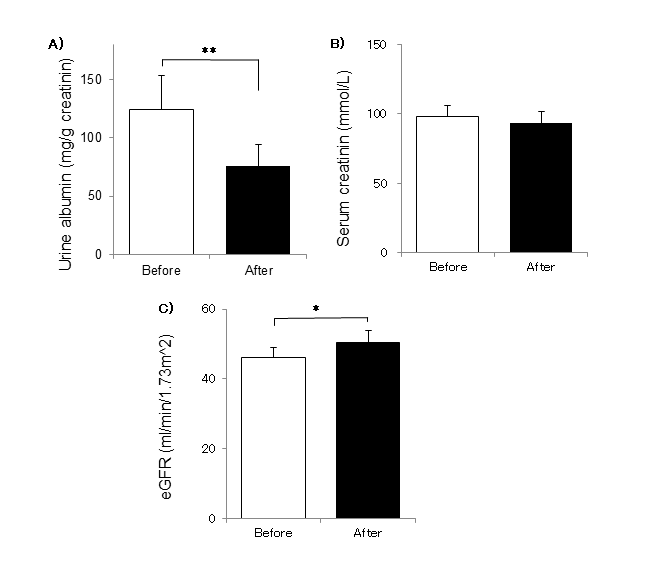
Figure 2.Changes of renal functions( Urinary albumin, Serum Creatinine, eGFR) A) Urine albumin, B)Serum creatinine C) eGFR. The results are expressed as means ± SE. (n=14). After the additional observation (3 months), the values significantly different from the initial(before) values (* p < 0.05*p<0.01 )
Other laboratory data
AST, ALT and γ-GTP decreased 10-30% % during the study period, but not significant.
Blood Pressure
Five systolic hypertensive subjects were included and 4 were normalized with the use of angiotensin receptor blocker (ARB) inhibitor. No significant change was observed during the intake period of supplement.
The mean systolic blood pressure decreased from 151 ± 1.6 mmHg to 136 ± 1.7 mmHg during the study period (p=0.175). The diastolic blood pressure slightly decreased from 80 ± 0.5 mmHg to 78 ± 1.8 mmHg (p=0.616).
Additional trial
As a follow-up observation after the test period, some male subjects continued to intake β-conglycinin with agreement without reducing protein intake (5g corresponding to β-conglycinin supplement) from regular food. Typical case was shown in Figure 3. Despite removing protein restrictions with supplementary β-conglycinin, both TG and LDL-C together with renal function showed improvement.
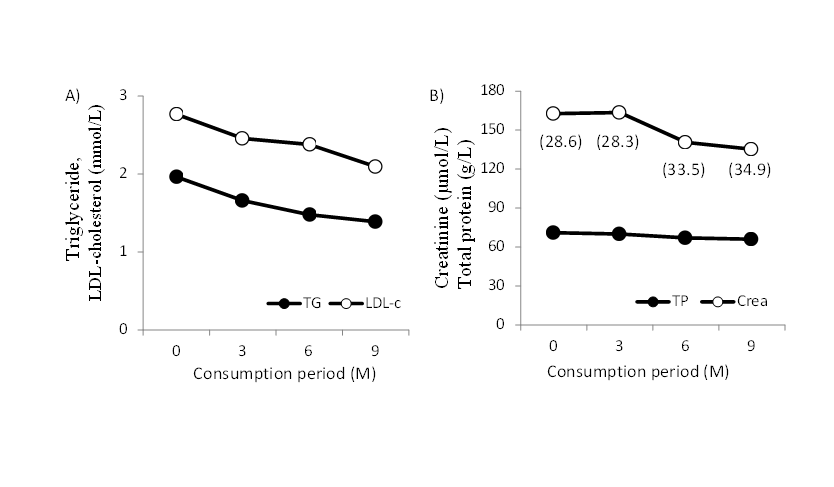
Figure 3.Typical case of long-term use of β-conglycinin supplement A) TG, triglyceride; LDL-C, LDL-cholesterol. B) TP, total protein; Crea, creatinine. Numbers in parentheses show eGFR (ml/min/1.73m2). Dietary guidance available for 0 to 3 months of intake period of β-conglycinin. No reduction of regular protein intake after 3 months.
Discussion
Statin and fibrate are drugs commonly used clinically to improve blood lipid levels. However, there are known side effects from statins, including muscle symptoms, rhabdomyolysis (secondary renal failure due to destruction of specific muscle tissue), peripheral neuropathy, myopathy, liver dysfunction, and thrombocytopenia [14-16]. Fibrate-related side effects include the development of slight gastric discomfort and myopathy (myalgia with the increased CPK). With rhabdomyolysis associated with elevated CPK, myoglobin in the muscle flows out into the blood and this myoglobin impairs the renal glomerular or tubular cells and induces kidney failure [17, 18]. Accordingly, it is necessary to carefully consider the pharmacokinetics of myogenic enzymes such as creatinine phosphokinase (CPK) and myoglobin at the time of administration of statins or fibrates, and combinational use of fibrate and statin for patients with renal dysfunction is contraindicated [19].
Therefore, supplementary use of β-conglycinin in these subjects either single or combinational use of stain is clinically important. This supplement is also useful for the weight reduction for obesity or subjects with metabolic syndrome replacing snacks rich in carbohydrate and fats.
Soy protein has been reported to suppress the progression of diabetic nephropathy [20-22] and have beneficial effect for correcting LDL-C [3, 23]. β-conglycinin is a major component of soy protein, has been reported to decrease serum triglyceride, LDL levels and visceral fat [1-4]. In this study, when chronic kidney disease patients with dyslipidemia ingested β-conglycinin for 3 months, triglyceride and LDL-cholesterol levels decreased without disturbing kidney function. Moreover, renal function of these patients improved. The mechanism of the renal favorable effect of β-conglycinin, is unknown. Overloading of lipid may impair renal function, which might have been corrected with β-conglycinin supplement.
CKD causes hypertension, which exacerbates existing CKD, forming a vicious circle. Since hypertension is a powerful risk factor for CVD (cardiovascular disease), antihypertensive therapy contributes to suppression of onset and progression of CVD. According to the guidebook of Japanese Society of Nephrology [9], the recommended blood pressure should be lower than 130/80 mmHg as the target for blood pressure reduction in the CKD. However, excessive depression may worsen kidney function; especially in elderly people aged 65 and older avoid pressure reduction to systolic blood pressure less than 110 mmHg. In this study, the mean blood pressure decreased from 151/80 to 136/78 during the study period without reducing renal function. The in vitro antihypertensive activity of the soy protein hydrolysates was compared by determining the angiotensin converting enzyme (ACE) inhibitory activity. Margatan et al. [24] reported that the papain-hydrolyzed β-conglycinin-rich fraction had ACE inhibitory activity, and β-conglycinin is a better precursor for antihypertensive peptides. Yang et al. [25] investigated the effects of soy protein and soy protein hydrolysate on blood pressure control, angiotensin-converting enzyme (ACE) activity, and renal function in a rat with chronic renal failure model. They reported that soy protein hydrolysate was effective in preventing the elevation of blood pressure, the progression of renal failure, and lowers kidney TNF-α level, plasma ACE activity, and insulin concentration. They concluded that the beneficial effects of soy protein on blood pressure and renal function may be mediated mostly by its pepsin-digested hydrolysate through its ACE inhibitory activity. These results show that β-conglycinin has a possibility to have beneficial effect for renal functions through blood pressure control.
In kidney disease, it is recommended that protein intake, including soy, should be carefully monitored and that patients should refrain from excessive protein intake. In this study, as a follow-up observation after the test period, some patients continued taking β-conglycinin removing the restriction of regular protein intake. As a result, even if the strict protein restriction was removed, in the case of addition of about 5 g of protein, blood lipids were controlled without adversely affecting renal function. There are several reports showing that mild protein restriction does not suppress the progression of kidney disease [26-28]. From now on, protein intake for patients whose kidney function is mildly deteriorated may be considered to need not merely a quantitative limitation of protein but management that takes quality into account. In fact, there are reports showing differences in the risk of incident CKD of animal proteins and plant proteins, including those derived from legumes, indicating the usefulness of plant proteins against the development of CKD [29-30]. Furthermore, the benefits of a plant-based diet for CKD, not only in terms of protein but also in terms of other components (fiber, plant fats, plant anions and plant phosphorus), have been noted [31].
We conclude that β-conglycinin may help to normalize lipid abnormalities in patients with renal dysfunction as a complementary use of β-conglycinin supplement, and improve renal dysfunction as a direct and/or secondary effect of ameliorating lipid abnormalities.
Conclusion
There is no appropriate drug for dyslipidemia in patients with kidney disease. These patients require dietary treatment for dyslipidemia. Soybean β-conglycinin has a beneficial ability to improve lipid metabolism, and in addition, it was also found to improve renal dysfunction. Soybean β-conglycinin is a food material that can play a supplementary role for lipid disorders in patients with reduced renal function.
Abbreviation
TG: Triglyceride; LDL-C: Low-density-lipoprotein cholesterol; Cr: serum creatinine; eGFR: estimated glomerular filtration rate; β-conglycinin: β-CG
Acknowledgements
This work is supported by grants from the Fuji Foundation for Protein Research. The authors greatly acknowledge the Japan Research Foundation for Healthy Aging for supporting clinical research for type 2 diabetes and lipid disorders.
References
1. Kohno M, Hirotsuka M, Kito M, et al. Decrease in serum triacylglycerol and visceral fat mediated by dietary soybean β-conglycinin. J Atheroscler Thromb. 2006; 13:247-255.
2. Ohara S, Matsui Y, Tamesada M, et al. Serum triacylglycerol-lowering effect of soybean β-conglycinin in mildly hypertriacylglycerolemic individuals. e-SPEN. 2007; 2:12-16.
3. Ma D, Taku K, Zhang Y, et al. Serum lipid-improving effect of soybean b-conglycinin in hyperlipidemic menopausal women. Br J Nutr.2013; 110: 1680–1684.
4. Nishimura M, Ohkawara T, Sato Y, et al. Improvement of Triglyceride Levels through the Intake of Enriched-β-Conglycinin Soybean (Nanahomare) Revealed in a Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2016;8(8):491. doi:10.3390/nu8080491.
5. Moriyama T, Kishimoto K, Nagai K, et al. Soybean β-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci Biotech Biochem. 2004; 68(2):352-359.
6. Fukui K, Kojima M, Tachibana N, et al. Effects of soybean β-conglycinin on hepatic lipid metabolism and fecal lipid excretion in normal adult rats. Biosci Biotech Biochem. 2004; 68(5):1153-1155.
7. Tachibana N, Iwaoka Y, Hirotsuka M, et al. β-conglycinin lowers very-low density lipoprotein-triglyceride levels by increasing adiponectin and insulin sensitivity in rats. Biosci Biotech Biochem. 2010; 74(6):1250-1255.
8. Saito T, Kohno M, Tsumura K, et al. Novel method using phytase for separating soybean β-conglycinin and glycinin. Biosci Biotech Biochem. 2001; 65:884–887.
9. Evidence-based Clinical Practice Guideline for CKD 2018 (in Japanese), Edited by Japanese Society of Nephrology, Tokyo Igakusya, 2018.
10. Guidelines for the management of obesity disease 2016 (in Japanese), Edited by Japan Society for the Study of Obesity. pp. 4-5, Life Science Publishing, 2016.
11. Guidelines for the prevention of atherosclerotic cardiovascular diseases 2017 (in Japanese), Edited by Japan Atherosclerosis Society, 2017.
12. Guidelines for the management of hypertension 2019 (in Japanese), Edited by High blood pressure treatment guideline preparation committee of Japan Hypertension Society. Life Science Publishing, 2019.
13. Guideline for the treatment for diabetes in Japan 2019 (in Japanese), Edited by the Japan Diabetes Society. Nankodo, 2019.
14. Thompson PD, Panza G, Zaleski A, et al. Statin-associated side effects. J Am Coll Cardiol. 2016, 67(20):2395-2410.
15. Taylor BA, Thompson PD. Statin-Associated Muscle Disease: Advances in Diagnosis and Management. Neurotherapeutics. 2018, 15(4):1006-1017.
16. Crisan E, Patil VK. Neuromuscular Complications of Statin Therapy. Curr Neurol Neurosci Rep. 2020, 20(10):47.
17. Kostapanos MS, Florentin M, Elisaf MS. Fenofibrate and the kidney: an overview. Eur J Cil Invest. 2013; 43(5): 522-532.
18. Kim S, Ko K, Park S, et al. Effect of fenofibrate medication on renal function. Korean J Fam Med. 2017; 38: 192-198.
19. Choi HD, Shin WG, Lee J-Y et al. Safety and efficacy of fibrate-statin combination therapy compared to fibrate monotherapy in patients with dyslipidemia: a meta-analysis. Vascul Phnamacol, 2015; 65-66:23-30.
20. Azadbakht L, Atbak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and c-reactive protein in type 2 diabetes with nephropathy. Diabetes Care. 2008; 31: 648-654.
21. Asanoma M, Tachibana N, Hirotsuka M, et al. Effects of soy protein isolate feeding on severe kidney damage in DOCA salt-treated obese Zucker rats. J Agric Food Chem. 2012; 60:5367-5372.
22. Jheng HG, Hirotsuka M, Goto Y, et al. Dietary low-fat soy milk powder retards diabetic nephropathy progression via inhibition of renal fibrosis and renal inflammation. Mol Nutr Food Res. 2016; 61(3):1600461.
23. Lamarche B, Desoches S, Jenkins D, et al. Combined effects of a dietary portfolio of plant sterols, vegetable protein, viscous fiber and almonds on LDL particle size. British J Nutr, 2004; 92: 657-663.
24. Margatan W, Rund K, Wang Q, et al. Angiotensin converting enzyme inhibitory activity of soy protein subjected to selective hydrolysis and thermal processing. J Agric Food Chem. 2013; 61: 3460−3467.
25. Yang HY, Chen JR, Chang LS. Effects of soy protein hydrolysate on blood pressure and angiotensin-converting enzyme activity in rats with chronic renal failure. Hypertens Res. 2008; 31:957–963.
26. Pan Y, Guo LL, Jin, HM. Low-protein diet for diabetic nephropathy: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008; 88:660-666.
27. Koya D, Haneda M, Inomata S, et al. Low-Protein Diet Study Group: Long-term effect of modification of dietary protein intake on the progression of diabetic nephropathy: a randomized control trail. Diabetologia. 2009; 52(10):2037-2045.
28. Rughooputh MS, Zeng R, Yao Y. Protein diet restriction slows chronic kidney disease progression in non-diabetic and in type 1 diabetic patients, but not in type 2 diabetic patients: a meta-analysis of randomized controlled trials using glomerular filtration rate as a surrogate. PLoS One. 2015; 10(12): e0145505.
29. Haring B, Selvin E, Liang M, et al. Dietary protein sources and risk for incident chronic kidney disease: Results from the atherosclerosis risk in communities (ARIC) study. J Ren Nutr. 2017;27(4):233-242.
30. Joshi S, McMacken M, Kalantar-Zadeh K, Plant-Based Diets for Kidney Disease: A Guide for Clinicians. Am J Kidney Dis. 2021; 77(2):287-296.
31. Carrero JJ, Gonzalez-Ortiz A, Avesani CM, et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat Rev Nephrol. 2020;16(9):525-542.
Received: September 14, 2020;
Accepted: September 28, 2020;
Published: October 04, 2020.
To cite this article : Ueda F, Ohtoshi K, Kohno M, et al. Effects and Clinical Significances of Orally Supplementary Soybean β-Conglycinin in Subjects with Dyslipidemia and Renal Dysfunction. British Journal of Gastroenterology. 2021; 3:1.
© Ueda F, et al. 2021.
