Research Article / Open Access
DOI:10.31488/bjg.1000129
Gut-Brain Axis and Food
Monjur Ahmed
1.Thomas Jefferson University Hospital, USA
*Corresponding author: Monjur Ahmed, MD, FRCP. Thomas Jefferson University Hospitals. 132 South 10th Street, Main Building, Suite 468, Philadelphia, Pennsylvania, 19107.
Abstract
The gut and brain communicate through the autonomic neurons, hormones, neurotransmitters, and microbial metabolites. The brain can sense the 'gut's feelings,' and the gut can respond to the brain's instructions. Gut microbiota plays a tremendous role in modulating the gut-brain axis in normal and abnormal states. They can produce hormones, neurotransmitters, vitamins, and many active metabolites, influencing brain growth, development, inflammation, and degeneration. Intestinal dysbiosis can lead to a variety of gastrointestinal and neurological diseases. Diet has a strong influence on modifying the gut microbiota. Dietary alteration can positively influence the gut-brain axis and our health.
Keywords: Gut-brain connection, microbiota-gut-brain axis, diet and gut-brain axis, microbiota and bowel diseases, microbiota and neurological diseases
Introduction
The term 'gut-brain axis' (GBA) was coined in the 1960s and 1970s when the amine and peptide-producing cells of the gut and the brain revealed several peptides [1]. Scientists have extensively studied the gut-brain axis concept in the last few decades. Recent studies suggest that the gut microbiota plays a significant role in maintaining the gut-brain axis. Food has a tremendous effect on the selection and growth of the microbiota [2]. The GBA is a bi-directional pathway between the brain and the gut as they are connected physically and biochemically in multiple ways. The subcortical region of the brain gets constant information from the gut and vice versa. The neural pathways, neuroendocrine, and neuroimmune systems establish the axis. [3]. Because of the extraordinary effects of microbiota on the brain-gut axis, the axis is also called the microbiota-gut-brain (MGB) axis. This article will discuss the different components of this axis, diseases related to this axis, and the role of food in this axis.
Neural pathway
The enteric nervous system (ENS) comprises the most significant part of the autonomic nervous system. It is a mesh-like neural network in the submucosa (Meissner's plexus) and muscularis propria (myenteric plexus) of the gut. The parasympathetic and sympathetic nervous systems constitute the other parts of the autonomic nervous system and originate from the CNS with their cell bodies in the brainstem and spinal cord. They have extensive connections with the ENS. The ENS has extensive bidirectional connections with the brain and spinal cord via the sympathetic and parasympathetic fibers [4]. The small intestinal and colonic ENS contains sensory neurons, interneurons, motor neurons, and entire reflex circuits, and is responsible for receiving sensory signals from the gut, regulation of intestinal motility, secretion, local blood flow, fluid, and electrolyte exchange between gut lumen and mucosa. Although the ENS constantly works in concert with the CNS, it can function as an independent entity without getting any signal from the brain. The Vagus nerve is the longest parasympathetic neuron that connects the brain (via nucleus of tractus solitarius and dorsal motor nucleus) and the gut (stomach, small intestine, and proximal part of the colon), innervating the myenteric plexus of the gut wall, and sends signals bi-directionally. The vagal afferent fibers carry the chemical and mechanosensory signals from the gut related to food intake. Vagal reflexes coordinate gastrointestinal motility and digestive functions through the neurotransmitter acetylcholine [5]. The distal colon and rectum are supplied by pelvic nerves, which are, in fact, sympathetic fibers [6]. Sympathetic neurons act on α- or β-adrenergic receptors in the gut wall via catecholamines and inhibit intestinal motility and secretion.
Neuroendocrine system
The gastrointestinal tract (GIT) works as the body's largest endocrine organ. The GIT secretes many hormones which influence brain activity. Ghrelin is an orexigenic hormone secreted from the ghrelinergic cells of the gastric fundus, small intestine and colon. The arcuate nucleus of the hypothalamus detects circulating ghrelin to initiate its orexigenic effect [7]. Gastric leptin is an anorexigenic hormone secreted from gastric chief cells. It binds to the arcuate nucleus of the hypothalamus and controls short-term food intake [8]. Substance P is a neuropeptide found in the myenteric plexus, vagus nerves, and CNS. It can bind to neurokinin-1 (NK-1) receptors in the vomiting center of the medulla oblongata and can cause nausea and vomiting [9]. Gastrin releasing peptide (GRP) is present in the enteric nervous system, hypothalamus, and brain stem. It acts as a universal on-switch stimulating gastrin secretion and most gastrointestinal hormones, intestinal and pancreatic secretions, and controls gut motility [10]. Somatostatin is an inhibitory hormone released from the hypothalamus and neuroendocrine delta (D) cells present in the pancreas and throughout the GIT. In the GIT, it works as a universal off-switch inhibiting the secretion of gastrin, secretin, cholecystokinin, insulin, glucagon, and vasoactive intestinal peptide (VIP). It also inhibits the release of corticotropin (ACTH), thyrotropin (TSH), and growth hormone (GH) from the pituitary gland [11]. The duodenal and jejunal I cells secrete cholecystokinin (CCK) in response to partially digested food. Besides stimulating gallbladder contraction, pancreatic enzyme secretion, and gut motility, it inhibits the release of ghrelin from the hypothalamus. It also binds to CCK-1 receptors in the hind-brain and induces satiety [12]. Neurotensin is widely distributed in the brain, and it is also secreted from the N cells present in the ileum and myenteric plexus of the enteric nervous system. Neurotensin acts as a neurotransmitter in the brain and inhibits dopaminergic pathways [13]. In the GIT, it stimulates gastric, intestinal, and pancreatic secretions, decreases gastric and small intestinal motility, increases colonic motility, and promotes small intestinal and colonic mucosal growth [14]. The origin, mechanism of action and effects of these hormones on the GBA are summarized in table 1.
The hypothalamic-pituitary-adrenal (HPA) axis is another bidirectional neuroendocrine pathway that balances our stress response. The HPA axis is also connected to the limbic system, essential for our emotions and memory. Cortisol is secreted from the adrenal cortex in response to stress. Excessive serum cortisol has a negative feedback effect on the HPA axis. Stress can also activate the sympathetic nervous system and adrenal medulla, which secretes catecholamines directly into the blood. During stress, the blood flow to the digestive tract is diverted to the brain, essentially shutting down digestion. The corticotropin-releasing hormone can directly increase the permeability of the mucosal epithelial lining of the gut, related to visceral hypersensitivity. The leaky gut mucosa can allow the passage of toxins and infectious agents from the gut lumen to the bloodstream, resulting in chronic low-grade inflammation of gut mucosa [15]. Stress can alter gut motility, gastrointestinal secretions, and visceral perception.
Certain diseases like irritable bowel syndrome (IBS) and other functional gastrointestinal disorders, inflammatory bowel disease (IBD), gastroesophageal reflux disease (GERD), and peptic ulcer disease (PUD) can flare up during stress. The gut microbiome can be affected directly by stress. Stress-induced sympathetic overactivity can suppress good bacteria in the gut and decrease gut immunity [16].
Table 1.Hormones affecting the GBA
| Origin | Mechanism of action | Effects | |
|---|---|---|---|
| Ghrelin | Ghrelinergic cells in gastric fundus, small intestine and colon. | Acts on arcuate nucleus of hypothalamus | Orexigenic |
| Gastric Leptin | Gastric chief cells | Binds to arcuate nucleus of hypothalamus | Anorexigenic |
| Substance P | Myenteric plexus, vagus nerves, and CNS. | Binds to neurokinin-1 (NK-1) receptors in the vomiting center of the medulla oblongata | Nausea and vomiting |
| GRP | Enteric nervous system, hypothalamus, and brain stem | Binds to GRP receptors in the GIT and brain | Stimulates the secretion of most gastrointestinal hormones and intestinal and pancreatic secretions and controls gut motility. |
| Somatostatin | Hypothalamus and neuroendocrine delta (D) cells present in the pancreas and throughout the GIT | Binds to somatostatin receptors in the GIT and brain. | Inhibits the secretion of gastrin, secretin, cholecystokinin, insulin, glucagon, VIP, GH, TSH and ACTH |
| CCK | Duodenal and jejunal I cells located within the crypts of Lieberkuhn. | Binds to CCK receptors in the GIT and brain | (1)Stimulates gallbladder contraction, pancreatic enzyme secretion, and gut motility. (2) Delays gastric emptying. (3) Inhibits the release of ghrelin from the hypothalamus. It also binds to CCK-1 receptors in the hind-brain and induces satiety. |
| Neurotensin | Widely distributed in the brain and N cells present in the ileum and myenteric plexus of the enteric nervous system. | Binds to neurotensin receptors in the GIT and brain. | In the GIT, (1) it stimulates gastric,
intestinal, and pancreatic secretions,
(2) decreases gastric and small
intestinal motility, (3) increases
colonic motility, and (4) promotes
small intestinal and colonic mucosal
growth. In the brain, it inhibits dopaminergic pathways. |
Neuroimmune system
The GIT contains many neuronal cells and immune cells. Peptidergic neurons play a unique role in impacting the mucosal immune system through the neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP). Immune cells secrete different cytokines and mediators like TNF-α and IL-1β, and neuronal cells express receptors for those [17]. Neuronal cells communicate with three innate immune cells in the gut: macrophages, innate lymphoid cells (ILC), and mast cells. ILCs are the first immune responders to intestinal tissue damage. The inflammatory response can alter intestinal permeability allowing endotoxin lipopolysaccharide (LPS) to release into the blood. LPS can cross the blood-brain barrier and increase the activity of the amygdala, which is involved in processing emotion (anxiety, aggression), motivation, memory, decision making, rewarding, threatening, and fearful environmental stimuli [18]. Mast cells are present in the mucosa and submucosa of the GIT. Mast cell degranulation releases histamine, serotonin, and tryptase, sensitizing the sensory neurons to manifest visceral pain. Neurotransmitters from sympathetic and parasympathetic neurons can regulate mast cell degranulation [19]. Vasoactive intestinal peptide (VIP) produced by mast cells and enteric neurons can also cause mast cell degranulation and play an essential role in the pathogenesis of irritable bowel syndrome [20].
Microbiota and brain-gut axis
Trillions of symbiotic microorganisms living in and on us are collectively called microbiota. Their genomes (also called the second genome) contain approximately 150 times the gene content as our genome. They are present on the skin and genital organs, oral cavity, respiratory tract, and gastrointestinal tract. But the colon and ileum harbor the densest concentration of microbiota. Research over the last few decades by Next Generation Sequencing (NGS) and high-performance liquid chromatography (HPLC) suggest that the gut microbiota play a key role in regulating the brain-gut axis, human physiology, health, and diseases. The human microbiota is predominantly composed of bacteria, but archea, bacteriophage, eukaryotes, fungi, protozoa, and viruses also inhabit as microbiota [21]. The three major bacterial phyla, as shown in table 2, are Firmicutes (79.4%), Bacteroidetes (16.9%), and Actinobacteria (2.5%) [22]. Firmicutes include Clostridium, Enterococcus, Lactobacillus, and Faecalibacterium genera. Bacteroidetes include Bacteroides and Prevotella genera.
Although the microbiomes start to colonize in our gut at birth, the adult state complex microbiomes are established at three years [23]. Gut microbial population in an individual depends on many factors like periconceptional maternal health and diet, mode of delivery, type of feeding during infancy, host genetics, age, stress, depression, infection, antibiotics intake, diet, and non-specific host factors, which include colonic epithelial secretion of mucus, antimicrobial peptides and immunoglobin A [24-27]. Human genes can influence the composition of gut microbiota [28]. Prebiotics, probiotics, and fecal microbiota transplantation (FMT) can modulate gut microbiota. Modulation of gut microbiota can interrupt the production of normal metabolites, and this can have a significant effect on host gene expression with lasting effects in the host [29]. The microbiota communicates with the brain through the neural, neuroendocrine, and neuroimmune systems and tryptophan metabolism [30]. Microbial metabolites like branched-chain amino acids (BCAA), short-chain fatty acids (SCFA), and peptidoglycans are involved in this process [31]. The microbiota plays a vital role in developing certain human diseases through the brain-gut axis. Colonic ammoniagenic bacteria in liver cirrhosis contribute to hepatic encephalopathy [32]. Changes in microbiota in IBS may lead to immune activation, low-grade inflammation, altered function of the enteric, autonomic, and central nervous system leading to visceral hypersensitivity, abnormal gut motility, and secretion [33]. The microbiota population in healthy individuals and patients with IBD is different. Many environmental factors like diet, antibiotics, and stress change the microbiota, leading to chronic intestinal inflammation due to the release of LPS and proinflammatory cytokines. The inflammation, in turn, can promote dysbiosis by altering the metabolic and oxidative environment of the gut [34]. Dysbiosis leads to increased intestinal permeability or leaky gut syndrome. The proinflammatory cytokines can interact with the CNS via enteric afferent neurons and the vagus nerve or directly through the blood-brain barrier. The cytokines can also activate the HPA axis with the release of corticosteroids involved in controlling emotion and cognition and the pathogenesis of anxiety, depression, and stress [35]. Stress has multiple effects on gut physiology, including increased visceral perception, increased intestinal permeability, reduced mucosal blood flow, changes in gut motility and secretion, and adverse effects on gut microbiomes. SCFA formed by microbial fermentation of starch and non-starch polysaccharides is the primary signaling molecule mediating host-microbiota communication. Butyrate can inhibit histone deacetylase and stimulate memory and synaptic plasticity [36]. It can influence enterochromaffin cells and promote colonic serotonin production [37]. Propionate has a protective effect on the blood-brain barrier from oxidative stress [38]. Certain gut bacteria can synthesize neuro-transmitters and hormones identical to those produced by human beings. These include acetylcholine and gamma-amino butyrate (GABA) synthesized by Lactobacillus and Bifidobacterium; and serotonin, dopamine, and norepinephrine produced by Streptococcus, Enterococcus, and Escherichia [39, 40]. They can act on the GBA through the enteric nervous and vagus nerve.
Table 2.Category of microbiota
| Microbiota | |||
|---|---|---|---|
| Phyla | Firmicutes | Bacteroidetes | Actinobacteria |
| Genera | Clostridium, Enterococcus, Lactobacillus, and Faecalibacterium | Bacteroides and Prevotella | Bifidobacterium |
Microbiota can also produce specific vitamins. These include vitamin K by Escherichia coli, Klebsiella pneumoniae, Propionibacterium, and Eubacterium; vitamin B2 by Bacillus subtilis and E. coli; folic acid by Bifidobacterium, Lactococcus lactis and Streptococcus thermophiles; and vitamin B12 by Lactobacillus reuteri and Propionibacterium freudenreichii [41]. Gut microbiomes can metabolize tryptophan to synthesize serotonin, melatonin, kynurenine, quinolinate, indole, indole acetic acid (IAA), indole propionic acid (IPA), and tryptamine [42]. Kynurenine and quinolinate can cause depression-like symptoms [43]. Indole acetic acid can cause cognitive impairment in hemodialysis patients [44]. As a result of the utilization of tryptophan by the bacteria, the host can have reduced serum concentration of tryptophan and reduced production of neurotransmitters, particularly serotonin in the brain, with the consequence of decreased serotonergic neurotransmission in the central and enteric nervous system and impaired cognition, fatigue, anxiety and depression [45]. Microbiota may have a role in developing and progressing neurodegenerative diseases like Alzheimer's disease and Parkinson's disease, and certain neurodevelopmental disorders, including attention deficit hyperactivity disorder (ADHD), autism, schizophrenia, and epilepsy due to chronic neuroinflammation and microglial activation. [46, 47]. A graphic representation of the mechanism by which the microbiota interacts with the GBA is shown in figure 1.
Food and brain-gut axis
Food has been found to have the most significant effect on the distribution and function of gut microbiota. Dietary intervention can modulate gut microbiota. Even short-term consumption of diet can rapidly alter the gut microbiota population. Certain groups of foods are more beneficial than others for the gut-brain axis.
An animal-based diet can increase bile-tolerant microbiota like Bacteroides, bilophila, and alistipes but can decrease firmicutes [48]. High saturated/trans-fat, low fiber, and refined carbohydrate diet can contribute to cognitive dysfunction, and even development and progression of Alzheimer's disease, possibly through the microbiota metabolites [49].
A plant-based diet contains linoleic acid metabolized by gut microbiota (certain intestinal bifidobacteria) to form conjugated linoleic acid (CLA). CLA has anti-inflammatory, anti-diabetogenic, anti-carcinogenic, and anti-adipogenic effects [50]. Phytochemicals and antioxidants in a plant-based diet can protect against cognitive decline and neurodegenerative diseases like dementia [51].
A high-fat diet can cause intestinal dysbiosis due to increased Firmicutes and decreased Bacteroides. This alteration has been associated with obesity, increased gut permeability, and chronic low-grade inflammation due to increased intestinal LPS-bearing bacterial species [52]. Mice study also showed that a high-fat diet could cause neuroinflammation, disrupting cognitive and stereotypical behavior and cerebrovascular homeostasis [53].
A high fiber diet (fruits, vegetables, grains, nuts, and seeds) contains carbohydrate polymers that are non-digestible and non-absorbable by our small bowel. Microbiomes can metabolize them to produce SCFA, which protects the blood-brain barrier and memory function, as mentioned before. SFCA can also increase brain-derived neurotrophic factor (BDNF) levels and influence cognition and affective processes [54]. A high fiber diet can restore maternal obesity-induced cognitive and behavioral disorders in the offspring through the effect of SCFA on the brain-gut axis [55].
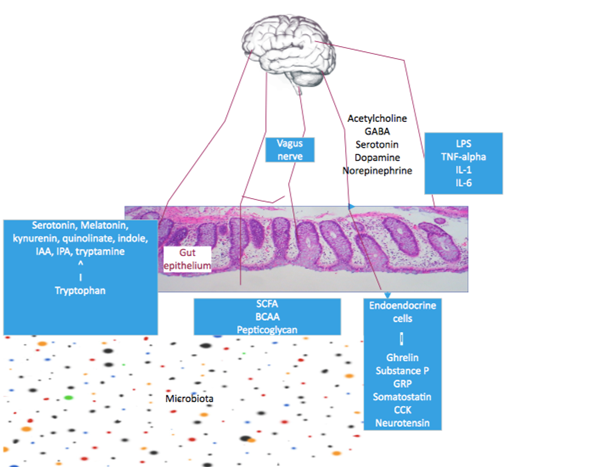
Figure 1.MGB
Mediterranean diet (MedDiet): is considered one of the healthiest and most sustainable diets. Vegetables, especially leafy greens, fresh fruits, olives, legumes, whole grains, nuts, seeds, extra-virgin olive oils, fish/seafood, spices, herbs, low-fat dairy in the form of cheese or yogurt, and red wine in moderation are the core food contents of MedDiet [56]. Daily intake of MedDiet changes the gut microbiota to an increased presence of Bacteroidetes, depletion of Firmicutes, lower ratio of Firmicutes/Bacteroidetes, decreased Escherichia coli (E.coli), an increased proportion of Bifidobacteria/E. coli and an increase in Proteobacteria [57- 59]. Besides cardiovascular, metabolic, and anti-cancer benefits, MedDiet can reduce gut inflammation, lower the risk of depression, decrease the incidence of Parkinson's disease (PD) and Alzheimer's disease (AD) [60, 61]. The current theory suggests that PD starts from the gut when gut microbial dysbiosis, particularly low levels of Provotella, causes increased intestinal inflammation; it allows exposure to bacterial endotoxin (locally and systemically) and provokes expression of alpha-synuclein in the colon. Misfolding alpha-synuclein leads to Lewy bodies that spread from the ENS to the CNS via the vagus nerve. Accumulation of the Lewy body in the substantia nigra leads to neurodegeneration and PD. MedDiet provides healthy microbiota, including Provotella, and prevents the development of PD [62]. Berti et al. found that people who ate MedDiet had fewer beta-amyloid deposits (a marker of AD) in their brain than those who ate a Western diet [63].
Other foods beneficial for the gut-brain axis
1. Fermented foods: include cheese, yogurt, kefir, and sauerkraut. They contain several lactic acid bacteria. They may improve cognitive function by normalization of stress-induced HPA axis hyperactivity. They may also enhance hippocampal expression of BDNF necessary for neuronal survival and differentiation [64].
2. Tryptophan-rich foods: include eggs, cheese, meat, fruits, and seeds. Gut microbiota can utilize tryptophan and synthesize serotonin from tryptophan. Serotonin acts as a neurotransmitter in the CNS and enteric nervous system. A high tryptophan diet (>10 mg/kg body weight/day) can decrease anxiety, depression, and irritability [65].
3. Polyphenol-rich foods: include coffee, green tea, olive oil, and cocoa. They can increase healthy gut microbiomes with decreased intestinal permeability and bacterial translocation into the bloodstream [66]. They also can improve cognition, memory, and learning [67].
4. Omega-3 polyunsaturated fatty acids (PUFAs): are found in oily fish, canola oil, soybean oil, flaxseed oil, nuts, seeds, grains, fruits, vegetables, and legumes. They can positively affect microbiota, decreasing firmicutes/Bacteroidetes ratio, increasing Prevotella genus, and thus increasing the production of short-chain fatty acids [68]. Omega-3 PUFAs are also an integral part of the neuronal membrane and have healthy neuronal functioning. Supplementation of Omega-3 PUFA may significantly affect mood and cognition [69]. The different food groups with their effects and mechanism of communication with the GBA are summarized in table 3.
Conclusion
Gut-brain axis is the bidirectional connection between the gut and the brain through the neural, neuroendocrine, and neuroimmune pathways. The gut can sense and signal the brain, and vice versa. Microbiota communicates with the axis by its metabolites and synthesizing and utilizing different neurotransmitters. Intestinal dysbiosis can increase gut permeability, inflammation, and translocation of proinflammatory cytokines to activate the CNS and HPA. Microbiota can also play a role in developing different functional and inflammatory bowel diseases, cognitive and affective disorders, neurodevelopmental, neurodegenerative, and autoimmune diseases. Diet is the most potent modifiable factor that can influence gut microbiota composition. Some diets can positively modulate the gut microbiota and prevent different human diseases. Most of the knowledge about the MGB axis comes from animal models. Further research is warranted in this field to have a more significant impact on human health.
Competing Interest Statement
None.
Table 3.Food groups with their effects and mechanism of communication with the GBA
| Diet | Effects and mechanism of communication with the GBA |
|---|---|
| Animal-based diet | Cognitive dysfunction through microbiota metabolites |
| Plant-based diet | Phytochemicals and antioxidants protect against cognitive decline |
| High-fat diet | Disrupts cognitive and stereotypical behavior and cerebrovascular homeostasis by causing neuroinflammation |
| High fiber diet | SCFA produced by gut microbiota protects the blood-brain barrier and memory function.
SCFA also improves cognition and affective function by increasing BDNF levels. |
| MedDiet | Decreases the risk of depression, PD and AD by providing healthy gut microbiota and reducing gut inflammation |
| Fermented foods | Improve cognitive function by normalization of stress-induced HPA axis hyperactivity |
| Tryptophan-rich foods | Can decrease anxiety, depression, and irritability due to the production of serotonin by gut microbiota |
| Polyphenol-rich foods | Can improve cognition, memory, and learning due to increased healthy gut microbiota, decreasing intestinal permeability. |
| Omega-3 polyunsaturated fatty acids | Being an integral part of the neuronal membrane improves mood and cognition |
Contributorship Statement
Monjur Ahmed, MD solely contributed to the work.
Funding Statement
This work received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
1. Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195-219. doi: 10.1007/978-1-4939-0897-4_9. PMID: 24997035; PMCID: PMC4359909.
2. Singh RK, Chang HW, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017 Apr 8;15(1):73. doi: 10.1186/s12967-017-1175-y. PMID: 28388917; PMCID: PMC5385025.
3. Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381-96. doi: 10.1146/annurev-med-012309-103958. PMID: 21090962; PMCID: PMC3817711.
4. Furness JB, Callaghan BP, Rivera LR, et al. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39-71. doi: 10.1007/978-1-4939-0897-4_3. PMID: 24997029.
5. de Jonge WJ. "The Gut’s Little Brain in Control of Intestinal Immunity",International Scholarly Research Notices, vol. 2013, Article ID 630159, 17 pages,2013. https://doi.org/10.1155/2013/630159
6. Espinosa-Medina I, Saha O, Boismoreau F, et al. The sacral autonomic outflow is sympathetic. Science. 2016 Nov 18;354(6314):893-897. doi: 10.1126/science.aah5454. PMID: 27856909; PMCID: PMC6326350.
7. Akamizu T, Iwakura H, Ariyasu H, et al. Ghrelin and functional dyspepsia. Int J Pept. 2010;2010:548457. doi: 10.1155/2010/548457. Epub 2010 Jan 12. PMID: 20721353; PMCID: PMC2915802.
8. Cammisotto P, Bendayan M. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol. 2012 Mar;45(1):1-16. doi: 10.5115/acb.2012.45.1.1. Epub 2012 Mar 31. PMID: 22536547; PMCID: PMC3328736.
9. Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63 Suppl 11:6-10. PMID: 12562137.
10. West SD, Mercer DW. Bombesin-induced gastroprotection. Ann Surg. 2005 Feb;241(2):227-31. doi: 10.1097/01.sla.0000151790.14274.5d. PMID: 15650631; PMCID: PMC1360124.
11. Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556-63. doi: 10.1056/NEJM198312223092506. PMID: 6140639.
12. Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2007 Feb;14(1):63-7. doi: 10.1097/MED.0b013e3280122850. PMID: 17940422.
13. Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999 Jul;20(7):302-9. doi: 10.1016/s0165-6147(99)01357-7. PMID: 10390649.
14. Yoshinaga K, Evers BM, Izukura M, Parekh D, Uchida T, Townsend CM Jr, Thompson JC. Neurotensin stimulates growth of colon cancer. Surg Oncol. 1992 Apr;1(2):127-34. doi: 10.1016/0960-7404(92)90025-g. PMID: 1341243.
15. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015 Oct 14;9:392. doi: 10.3389/fncel.2015.00392. PMID: 26528128; PMCID: PMC4604320.
16. Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011 Dec;62(6):591-9. PMID: 22314561.
17. Khalil M, Zhang Z, Engel MA. Neuro-Immune Networks in Gastrointestinal Disorders. Visc Med. 2019 Mar;35(1):52-60. doi: 10.1159/000496838. Epub 2019 Feb 4. PMID: 31312651; PMCID: PMC6597910.
18. Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015 Jan 15;517(7534):284-92. doi: 10.1038/nature14188. PMID: 25592533; PMCID: PMC4565157.
19. van Diest SA, Stanisor OI, Boeckxstaens GE, et al. Relevance of mast cell-nerve interactions in intestinal nociception. Biochim Biophys Acta. 2012 Jan;1822(1):74-84. doi: 10.1016/j.bbadis.2011.03.019. Epub 2011 Apr 7. PMID: 21496484.
20. Forsythe P. Mast Cells in Neuroimmune Interactions. Trends Neurosci. 2019 Jan;42(1):43-55. doi: 10.1016/j.tins.2018.09.006. Epub 2018 Oct 4. PMID: 30293752.
21. Kho ZY, Lal SK. The Human Gut Microbiome - A Potential Controller of Wellness and Disease. Front Microbiol. 2018 Aug 14;9:1835. doi: 10.3389/fmicb.2018.01835. PMID: 30154767; PMCID: PMC6102370.
22. Tap J, Mondot S, Levenez F, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009 Oct;11(10):2574-84. doi: 10.1111/j.1462-2920.2009.01982.x. Epub 2009 Jul 6. PMID: 19601958.
23. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012 May 9;486(7402):222-7. doi: 10.1038/nature11053. PMID: 22699611; PMCID: PMC3376388.
24. House JS, Mendez M, Maguire RL, et al. Periconceptional Maternal Mediterranean Diet Is Associated With Favorable Offspring Behaviors and Altered CpG Methylation of Imprinted Genes. Front Cell Dev Biol. 2018 Sep 7;6:107. doi: 10.3389/fcell.2018.00107. PMID: 30246009; PMCID: PMC6137242.
25. Morais LH, Golubeva AV, Moloney GM, et al. Enduring Behavioral Effects Induced by Birth by Caesarean Section in the Mouse. Curr Biol. 2020 Oct 5;30(19):3761-3774.e6. doi: 10.1016/j.cub.2020.07.044. Epub 2020 Aug 20. PMID: 32822606.
26. Coker MO, Laue HE, Hoen AG, et al. Infant Feeding Alters the Longitudinal Impact of Birth Mode on the Development of the Gut Microbiota in the First Year of Life. Front Microbiol. 2021 Apr 7;12:642197. doi: 10.3389/fmicb.2021.642197. PMID: 33897650; PMCID: PMC8059768.
27. Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019 Aug 16;7:e7502. doi: 10.7717/peerj.7502. PMID: 31440436; PMCID: PMC6699480.
28. Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell. 2014 Nov 6;159(4):789-99. doi: 10.1016/j.cell.2014.09.053. PMID: 25417156; PMCID: PMC4255478.
29. Nichols RG, Davenport ER. The relationship between the gut microbiome and host gene expression: a review. Hum Genet. 2021 May;140(5):747-760. doi: 10.1007/s00439-020-02237-0. Epub 2020 Nov 22. PMID: 33221945; PMCID: PMC7680557.
30. Martin CR, Osadchiy V, Kalani A, et al. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018 Apr 12;6(2):133-148. doi: 10.1016/j.jcmgh.2018.04.003. PMID: 30023410; PMCID: PMC6047317.
31. Cryan JF, O'Riordan KJ, Cowan CSM, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019 Oct 1;99(4):1877-2013. doi: 10.1152/physrev.00018.2018. PMID: 31460832.
32. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010 Mar 25;362(12):1071-81. doi: 10.1056/NEJMoa0907893. PMID: 20335583.
33. Ohman L, Simrén M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007 Mar;39(3):201-15. doi: 10.1016/j.dld.2006.10.014. Epub 2007 Jan 30. PMID: 17267314.
34. Ni J, Wu GD, Albenberg L, et al. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017 Oct;14(10):573-584. doi: 10.1038/nrgastro.2017.88. Epub 2017 Jul 19. PMID: 28743984; PMCID: PMC5880536.
35. Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608-17. doi: 10.1111/j.1749-6632.2000.tb05426.x. PMID: 11268389.
36. Vecsey CG, Hawk JD, Lattal KM, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007 Jun 6;27(23):6128-40. doi: 10.1523/JNEUROSCI.0296-07.2007. PMID: 17553985; PMCID: PMC2925045.
37. Reigstad CS, Salmonson CE, Rainey JF 3rd, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015 Apr;29(4):1395-403. doi: 10.1096/fj.14-259598. Epub 2014 Dec 30. PMID: 25550456; PMCID: PMC4396604.
38. Hoyles L, Snelling T, Umlai UK, Nicholson JK, Carding SR, Glen RC, McArthur S. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome. 2018 Mar 21;6(1):55. doi: 10.1186/s40168-018-0439-y. PMID: 29562936; PMCID: PMC5863458.
39. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012 Oct;13(10):701-12. doi: 10.1038/nrn3346. Epub 2012 Sep 12. PMID: 22968153.
40. Galland L. The gut microbiome and the brain. J Med Food. 2014 Dec;17(12):1261-72. doi: 10.1089/jmf.2014.7000. PMID: 25402818; PMCID: PMC4259177.
41. Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11(2):135-157. doi: 10.1080/19490976.2019.1638722. Epub 2019 Aug 1. PMID: 31368397; PMCID: PMC7053956.
42. O'Mahony SM, Clarke G, Borre YE, et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015 Jan 15;277:32-48. doi: 10.1016/j.bbr.2014.07.027. Epub 2014 Jul 29. PMID: 25078296.
43. Oxenkrug G. Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Curr Drug Targets. 2013 May 1;14(5):514-21. doi: 10.2174/1389450111314050002. PMID: 23514379; PMCID: PMC3726541.
44. Lin YT, Wu PH, Lee HH, et al. Indole-3 acetic acid increased risk of impaired cognitive function in patients receiving hemodialysis. Neurotoxicology. 2019 Jul;73:85-91. doi: 10.1016/j.neuro.2019.02.019. Epub 2019 Feb 28. PMID: 30826344.
45. Jenkins TA, Nguyen JC, Polglaze KE, et al. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients. 2016 Jan 20;8(1):56. doi: 10.3390/nu8010056. PMID: 26805875; PMCID: PMC4728667.
46. Larroya A, Pantoja J, Codoñer-Franch P, et al. Towards Tailored Gut Microbiome-Based and Dietary Interventions for Promoting the Development and Maintenance of a Healthy Brain. Front Pediatr. 2021 Jul 1;9:705859. doi: 10.3389/fped.2021.705859. PMID: 34277527; PMCID: PMC8280474.
47. Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015 Mar;30(3):350-8. doi: 10.1002/mds.26069. Epub 2014 Dec 5. PMID: 25476529.4
48. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014 Jan 23;505(7484):559-63. doi: 10.1038/nature12820. Epub 2013 Dec 11. PMID: 24336217; PMCID: PMC3957428.
49. Frausto DM, Forsyth CB, Keshavarzian A, et al. Dietary Regulation of Gut-Brain Axis in Alzheimer's Disease: Importance of Microbiota Metabolites. Front Neurosci. 2021 Nov 19;15:736814. doi: 10.3389/fnins.2021.736814. PMID: 34867153; PMCID: PMC8639879.
50. O'Shea EF, Cotter PD, Stanton C, et al. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012 Jan 16;152(3):189-205. doi: 10.1016/j.ijfoodmicro.2011.05.025. Epub 2011 Jun 14. PMID: 21742394.
51. Libro R, Giacoppo S, Soundara Rajan T, et al. Natural Phytochemicals in the Treatment and Prevention of Dementia: An Overview. Molecules. 2016 Apr 21;21(4):518. doi: 10.3390/molecules21040518. PMID: 27110749; PMCID: PMC6274085.
52. Murphy EA, Velazquez KT, Herbert KM. Influence of high-fat diet on gut microbiota: a driving force for chronic disease risk. Curr Opin Clin Nutr Metab Care. 2015 Sep;18(5):515-20. doi: 10.1097/MCO.0000000000000209. PMID: 26154278; PMCID: PMC4578152.
53. Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015 Apr 1;77(7):607-15. doi: 10.1016/j.biopsych.2014.07.012. Epub 2014 Jul 18. PMID: 25173628; PMCID: PMC4297748.
54. La Torre D, Verbeke K, Dalile B. Dietary fibre and the gut–brain axis: Microbiota-dependent and independent mechanisms of action. Gut Microbiome. 2021; 2, E3. doi:10.1017/gmb.2021.3
55. Liu X, Li X, Xia B, et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021 May 4;33(5):923-938.e6. doi: 10.1016/j.cmet.2021.02.002. Epub 2021 Mar 1. PMID: 33651981.
56. Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995 Jun;61(6 Suppl):1402S-1406S. doi: 10.1093/ajcn/61.6.1402S. PMID: 7754995.
57. Krznarić Ž, Vranešić Bender D, Meštrović T. The Mediterranean diet and its association with selected gut bacteria. Curr Opin Clin Nutr Metab Care. 2019 Sep;22(5):401-406. doi: 10.1097/MCO.0000000000000587. PMID: 31232713.
58. Mitsou EK, Kakali A, Antonopoulou S, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017 Jun;117(12):1645-1655. doi: 10.1017/S0007114517001593. PMID: 28789729.
59. Pisanu S, Palmas V, Madau V, et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients. 2020 Sep 4;12(9):2707. doi: 10.3390/nu12092707. PMID: 32899756; PMCID: PMC7551852.
60. Sánchez-Villegas A, Martínez-González MA, Estruch R, et al. Mediterranean dietary pattern and depression: the PREDIMED randomized trial. BMC Med. 2013 Sep 20;11:208. doi: 10.1186/1741-7015-11-208. PMID: 24229349; PMCID: PMC3848350.
61. Sofi F, Cesari F, Abbate R, et al. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008 Sep 11;337:a1344. doi: 10.1136/bmj.a1344. PMID: 18786971; PMCID: PMC2533524.
62. Uyar GÖ, Yildiran H. A nutritional approach to microbiota in Parkinson's disease. Biosci Microbiota Food Health. 2019;38(4):115-127. doi: 10.12938/bmfh.19-002. Epub 2019 Jun 29. PMID: 31763115; PMCID: PMC6856517.
63. Berti V, Walters M, Sterling J, et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018 May 15;90(20):e1789-e1798. doi: 10.1212/WNL.0000000000005527. Epub 2018 Apr 13. PMID: 29653991; PMCID: PMC5957301.
64. Kim B, Hong VM, Yang J, et al. A Review of Fermented Foods with Beneficial Effects on Brain and Cognitive Function. Prev Nutr Food Sci. 2016 Dec;21(4):297-309. doi: 10.3746/pnf.2016.21.4.297. Epub 2016 Dec 31. PMID: 28078251; PMCID: PMC5216880.
65. Lindseth G, Helland B, Caspers J. The effects of dietary tryptophan on affective disorders. Arch Psychiatr Nurs. 2015 Apr;29(2):102-7. doi: 10.1016/j.apnu.2014.11.008. Epub 2014 Dec 9. PMID: 25858202; PMCID: PMC4393508.
66. Guglielmetti S, Bernardi S, Del Bo' C, et al. Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: study protocol of the MaPLE randomised controlled trial. BMC Geriatr. 2020 Feb 26;20(1):77. doi: 10.1186/s12877-020-1472-9. PMID: 32102662; PMCID: PMC7045478.
67. Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev. 2012;2012:914273. doi: 10.1155/2012/914273. Epub 2012 Jun 3. PMID: 22701758; PMCID: PMC3372091.
68. Costantini L, Molinari R, Farinon B, et al. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int J Mol Sci. 2017 Dec 7;18(12):2645. doi: 10.3390/ijms18122645. PMID: 29215589; PMCID: PMC5751248.
69. Martí Del Moral A, Fortique F. Omega-3 fatty acids and cognitive decline: a systematic review. Nutr Hosp. 2019 Aug 26;36(4):939-949. English. doi: 10.20960/nh.02496. PMID: 31215788.
Received: April 04, 2022;
Accepted: April 25, 2022;
Published: April 27, 2022.
To cite this article : Monjur Ahmed. Gut-Brain Axis and Food. British Journal of Gastroenterology. 2022; 4(1): 242-248. doi: 10.31488/bjg.1000129
© Ahmed M. 2022.
