Research Article / Open Access
DOI:10.31488/bjg.1000128
Long Term Effect of Simvastatin in Primary Sclerosing Cholangitits: A Placebo-Controlled, Double-Blind, Multicenter Phase III Study (Piscatin)
Annika Bergquist*1, Hanns-Ulrich Marschall2, Emma Nilsson3, Nils Nyhlin4, Mårten Werner5, Amanda Klein1, Matteo Bottai6, Stergios Kechagias7, Fredrik Rorsman8
1.Department of Medicine Huddinge, Unit of Gastroenterology and Rheumatology, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden
2.Department of Molecular and Clinical Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
3.Unit ofGastroenterology and Hepatology, Skåne University Hospital, Sweden
4.Department of Gastroenterology, Faculty of Medicine and Health, Örebro University, Örebro, Sweden
5.Department of Public Health and clinical medicine, Umeå University, Umeå, Sweden
6.Division of Biostatistics, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden
7.Department of Health, Medicine and Caring Sciences, Unit of Internal Medicine, Linköping University, Linköping, Sweden
8.Fredrik Rorsman, Department of Gastroenterology and Hepatology, University Hospital, Uppsala, Sweden
*Corresponding author: Annika Bergquist, Department of Medicine Huddinge, Unit of Gastroenterology and Rheumatology, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden
Abstract
Background: Primary sclerosing cholangitis (PSC) is a chronic progressive liver disease with a variable disease course and high risk of premature death, need of liver transplantation (LT), and biliary malignancy. Currently, there is no pharmacological treatment that can halt PSC progression. Treatment with statins is beneficial in other chronic liver diseases and association between statin use and a favorable prognosis in PSC has been suggested. Aim: To evaluate the effect of simvastatin in PSC. Methods: PiSCATIN is a phase III, long-term randomized double-blind controlled trial of 40 mg simvastatin versus placebo in patients with PSC. The lack of reliable surrogate markers for disease progression that can serve as surrogate endpoints in PSC trials, motivates the primary composite endpoint of the first event of LT, biliary malignancy, variceal bleeding or death. Seven hundred patients from 14 centers in Sweden will be recruited and treated for 5 years. The study size and treatment duration to achieve adequate power were determined based on hypothesis generating Swedish register data, suggesting a halved risk for the composite endpoint after statin exposure, and data from a natural history Swedish cohort. Conclusions: PiSCATIN is the first long term randomized controlled study in PSC since 2009 and is designed to assess the effect of simvastatin on hard outcomes in PSC. The composite endpoint of biliary malignancy, liver transplantation, variceal bleeding or death is clinically the most relevant and will give robust evidence of the efficacy of simvastatin in PSC.
Introduction
Primary sclerosing cholangitis (PSC) is a chronic, progressive cholestatic liver disease affecting mostly young individuals, closely associated with inflammatory bowel disease (IBD) [1]. The prevalence of PSC is around 1 per 10,000 which means that PSC meets the criteria for the European union definition of a rare disease, defined as a prevalence of <5 per 10,000 inhabitants [2]. The disease course is highly variable. Increasing hepatobiliary fibrosis causes stricturing of the biliary tree which develops over years or decades leading to cirrhosis, end-stage liver disease, and need of liver transplantation (LT). Median time from diagnosis to death or LT is 21 years or longer, in the population-based setting [3]. Patients with PSC also have an increased risk for hepatobiliary and colorectal cancers [4].
There is no known medical treatment that can halt disease progression or decrease the risk of malignancy. The most extensively studied drug is ursodeoxycholic acid (UDCA), which is widely used, but the evidence in favor of UDCA is scarce [5, 6]. Other drugs including immunosuppressants, anti–inflammatory drugs, and antibiotics failed to show favorable effects [5].
Clinical trials in PSC are challenging. In addition to being rare, PSC is a slowly progressing disease with a great heterogeneity and low event rate of clinically relevant endpoints. There is an urgent need for prognostic tools that can be used as relevant surrogate endpoints for evaluation of new PSC treatments [7]. Moreover, large high quality natural history cohorts that can serve as controls for the regulatory authorities are currently missing.
Understanding the disease pathogenesis is essential for the selection of potential treatments. Progress has been made, but so far, triggering mechanisms of the disease in the genetic susceptible individual, the heterogeneity of the disease, and factors responsible for disease progression and risk for biliary malignancies remain unclear. Therefore, we decided to address this challenge by using hypothesis generating epidemiological data. In a population-based cohort study of 2,914 Swedish patients with PSC between 2005 and 2014, we searched for associations between the use of different drugs and PSC prognosis. Data from the Prescribed Drug Register was used to assess drug exposures, and the Patient Register, the Death Certificate Register and the Cancer Register were used to assess outcomes of interest (death, LT, variceal bleeding, and hepatobiliary cancer). Among all the studied drugs, statins were shown to be associated with the lowest risk of death or LT, hazard ratio (HR) 0.50 (95%CI 0.28-0.66) [8]. There is emerging evidence that the use of statins is beneficial in chronic liver disease by lowering the risk for liver failure and reducing mortality [9]. In addition, statins may also reduce the risk for bile duct malignancies [10].
Based on this, we initiated a placebo controlled randomized study with the aim to evaluate if simvastatin can prolong survival free of cancer, variceal bleeding, and LT.
Materials and Methods
Study design
This study is a randomized double-blind controlled study (PiSCATIN) in patients with PSC with a 1:1 randomization between simvastatin 40 mg once daily and placebo. All patients will be treated for 5 years or until occurrence of the composite endpoint; LT, hepatobiliary cancer, variceal bleeding, or death. Patients are followed-up according to the general clinical practice with yearly visits and sampling of liver function tests every six months. Three-hundred fifty patients in each treatment arm, 700 patients in total, will be included. The study is performed in a population-based setting with broad inclusion criteria which enables most of all patients with a confirmed diagnosis of PSC to participate in the trial. Inclusion and exclusion criteria are summarized in table 1. An overview of the study design is shown in figure 1.
Table 1.Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Verified PSC by cholangiography | Secondary sclerosing cholangitis |
| Age ≥18 and ≤75 years | On waiting list for liver transplantation |
| Patient’s informed consent | Child-Pugh ≥9 points |
| MR/MRCP performed within 4 months | Previous liver transplantation, variceal bleeding or hepatobiliary malignancy |
| Contraceptive use for fertile women | Serum CK >5 x ULN |
| If known IBD, colonoscopy performed within 24 months | Ongoing statin use |
| Intolerance against simvastatin | |
| Pregnancy or breast-feeding |
CK – creatin kinase; ULN- upper limit of normal
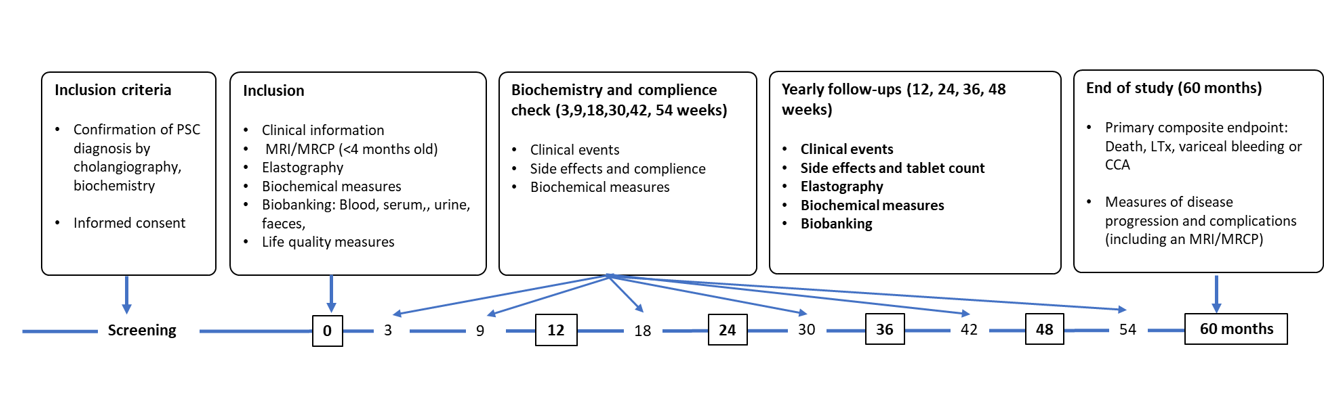
Figure 1.Overview of the study
Ethics
The PiSCATIN study has been approved by the Swedish Ethical Review Authority (2018/2462-31) and by the Swedish Medical Products Agency (EudraCT 2018-200814- 39). The ClinicalTrials.gov identifier for PiSCATIN is NCT04133792.
Endpoint rationale
Because of the slow rate of progression in PSC, assessment of hard clinical endpoints is a long-term endeavor. The lack of accepted surrogate endpoints to predict clinical benefit of medical treatment in PSC makes a study with a long-term follow-up and such hard endpoints motivated. The composite endpoint of LT, biliary malignancy, variceal bleeding or death is the most relevant composite endpoint for long-term treatment response and was used in previous large studies of UDCA in PSC [11, 12]. The PiSCATIN study may not only create evidence for the potential benefit of simvastatin, but also contribute to prospective data on natural history that may be of use as a control population for future open studies of new drugs. Sweden is one of the highest prevalence areas for PSC and therefore gives a unique opportunity to study PSC [13].
Since 2012 a prospective observational natural history study in PSC has been ongoing in Sweden including more than 500 patients followed for five years (ClinicalTrials.gov Identifier: NCT03041662). This observational study shows the feasibility for the prospective PiSCATIN study and provided useful data to perform power calculations.
Evaluation of secondary endpoints and biobanking for future evaluation of surrogate markers are also important. The following variables are measured at baseline, at every yearly visit, and at the end of study: serum alkaline phosphatase (ALP), serum bilirubin, MELD score, Child Pugh Score, FIB-4, MRI variables, liver stiffness by vibration controlled transient elastography (VCTE), PSC-related symptoms, dysplasia in gallbladder or bile ducts, colon cancer or dysplasia, and life quality measures. These parameters will all serve as secondary endpoints. The primary and secondary endpoints are detailed in Table 2.
Table 1.Primary and secondary endpoints
| Primary endpoint | |
|---|---|
| Clinical outcomes composite endpoint | Time to first occurrence of any of the following events: |
| a. Death | |
| b. Listed for LT | |
| c. First variceal bleeding | |
| d. Diagnosis of biliary malignancy or HCC | |
| Secondary endpoints | |
| Surrogate markers for treatment efficacy | a. Serum ALP |
| b. Serum bilirubin | |
| c. Liver stiffness | |
| Markers for PSC progression | |
| a. Fibrosis measures (FIB-4) * | |
| b. MELD score | |
| c. MRI parameters | |
| d. Clinical risk scores | |
| e. Child Pugh Score | |
| f. PSC-related symptoms** | |
| g. Quality of life*** | |
| h. Development of colon cancer or dysplasia |
*in addition, biobanked serum for additional analysis (ELF test or others)
**pruritus, bacterial cholangitis requiring treatment, ascites, encephalopathy
*** SF-36, PBC-40, CLDQ, IBDQ (in patients with IBD) and FQ-Meal-Q
(in 300 of the patients)
A consensus process in the International PSC Study Group (IPSCSG) concludes, although evidence is not convincing, that ALP levels, VCTE, and histology are the most relevant existing biomarkers for disease progression that may serve as surrogate endpoints in clinical trials [14]. Histology is an invasive procedure associated with some risks and discomfort for the patient. From our experience it is very difficult to motivate patients for repeated liver biopsies. Therefore, we included only ALP and VCTE as part of the secondary endpoints. Recent data from the simtuzumab study by Muir. et al., however, shows that ALP is hampered by huge intra-and inter-individual spontaneous variation which makes this marker unreliable [15, 16]. The best fibrosis marker that can serve as a surrogate endpoint in PSC seems to be enhanced liver fibrosis score (ELF)(17) which has less variability than ALP [16]. Due to high cost and the academic setting in which this study is performed, ELF is not measured up-front, although biobanked material will be available for post-hoc analysis.
MRI variables are possible future markers of importance for prognosis and evaluation of therapeutic effect in clinical trials in PSC. ANALI scores are MRI based scores that can be assessed both with and without contrast-enhanced investigations [18, 19] and are associated with outcomes. There is a high variability between readers but still ANALI scores seem to be useful markers for disease progression and will therefore be used as a surrogate secondary endpoint marker in this study [20].
Study interventions – follow-up
A long-term study of five years is a challenge. The risk for lack of motivation both in patients and physicians over time may increase risk for a high drop-out rate. To reduce this risk, we designed this study to be as close to regular clinical follow-up as possible. Annual follow-ups are the standard for PSC patients without severe disease. Therefore, yearly visits are performed, and a closer follow-up is done based on individual judgement by the responsible physician.
At inclusion, a recent MRI/MRCP (performed within four months) is required to confirm the diagnosis and to exclude hepatobiliary malignancy. Baseline evaluation includes registration of disease characteristics, previous events related to PSC or IBD, symptoms, analysis of liver function tests, clinical scores, and biobanking. All concomitant medications are registered. Patients are randomized to placebo or simvastatin using ALEA Tools for Clinical Trials. The electronic case report form (eCRF) uses the web- based Castor EDC clinical data management platform to ensure quality of the data.
A three-month follow-up visit (phone or video) with analysis of liver function tests is performed for potential side-effects and thereafter between the yearly visits (at 9, 18, 30, 42, 54 months) to improve compliance. Variables are collected in total at 12 occasions during the study, at baseline and at 3, 9, 12, 18, 24, 30, 36, 42, 48, 54, and 60 months (Figure 1). An MRI/MRCP is performed at the end of the trial in all patients.
Additional imaging data performed for clinical reasons during the study are registered but not included in the study protocol.
Rationale for dose of simvastatin
The rationale for choosing simvastatin is based on its high intrinsic HMG-CoA-reductase inhibition, low frequency of side effects, and data on safety for treatment for chronic liver disease/cirrhosis [21-23]. The dose was decided to 40 mg/day, with the possibility to lower the dose to 20 mg/day for patients experiencing side effects. A dose of 40 mg/day has been used in clinical trials evaluating simvastatin’s effect on cirrhosis without any cases of clinical rhabdomyolysis in compensated cirrhosis [22, 23]. In the LiverHope study [24] the side effects were frequent (19%) in patients with decompensated cirrhosis (Child B and C). PSC patients with Child B cirrhosis with ≥9 points are therefore not recruited in PiSCATIN. The ongoing phase III, double-blind, randomized clinical trial (SACRED) that investigates if simvastatin reduces incident hepatic decompensation in cirrhosis also uses the dose of 40 mg simvastatin [25].
Sample size and study duration justification
Sample size calculations were made assuming that simvastatin will halve the risk of a primary endpoint, which is based on projections from the risk estimates generated in our hypothesis generating register study [8]. Statins were shown to be associated with a reduced risk of death or LT, HR 0.50 (95%CI 0.28-0.66). The HR for the composite endpoint LT, hepatobiliary cancer, bleeding from esophageal varices or death caused by liver disease was 0.53 (0.36-0.80). The effect size of statins in the register study is comparable with the assumption of the effect of UDCA previously used in PSC [11]. We calculated the power of the log-rank test in a two-arm clinical trial to detect a HR of 0.5 at the two-sided level of 0.05. Based on natural history data from a national prospective observational study (ClinicalTrials.gov Identifier: NCT03041662) with the primary aim to evaluate MRI surveillance in PSC we assume that 440 patients are available at the start of the study and 120 more patients will be recruited annually over a two-year period. The survival in the reference group is estimated from historic data in this study with survival time defined as the time from study entry to the earliest of the following events: death, LT, or biliary malignancy (Figure 2). The probability of an event, required in the power calculation, was obtained with the Schoenfeld’s method and the estimated power was 0.804 when the total study time was set to 5 years (Figure 2).
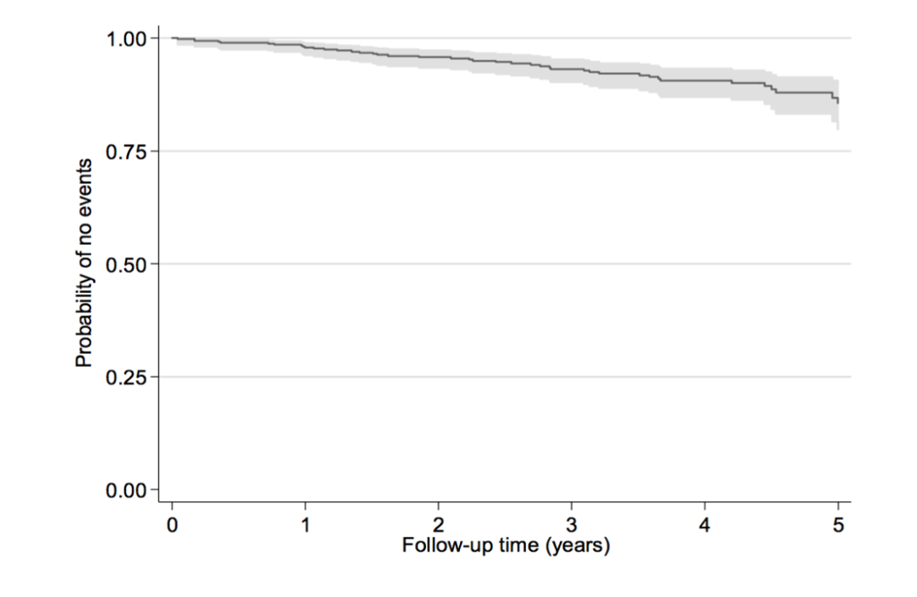
Figure 2.Probability of not experiencing death, transplant, or cancer diagnosis from a Swedish natural history cohort data 2012-2017 and estimation of study time in relation estimated power given that 680 patients are studied.
Statistical plan and data analyses
The Biostatistics Core Facility at Karolinska Institutet, Sweden is responsible for the correct execution. Any errors and inconsistency will be corrected, whenever possible. Patients’ compliance to treatment at each time-point, as measured by the tablet counts, is reported. The analyses will be performed under the intention-to-treat approach.
For evaluation of the effect of simvastatin on the primary endpoint (LT, biliary malignancy, variceal bleeding or death) we define a time-to-event variable as time in days elapsing between randomization to the earliest among time at death, LT, biliary malignancy, variceal bleeding, and end of follow-up. Those who have not experienced any of these three events at the end of follow-up are considered right censored observations. Because the end of follow-up date is set by design, we assume censoring does not carry any information on the time-to-event variable. We test the null hypothesis that the event-free survival function is equal between the two treatment groups with a two-sided log-rank test at the level of 0.05. Because of randomization, we assume the potential measured and unmeasured confounders to be equally distributed between the two treatment groups, bar random variability. Possible remaining unbalance at baseline is tested by the chi-squared test for categorical variables and the Kruskal-Wallis’ test for numeric variables. The number of missing values in the baseline measures and possible loss to follow-up is expected to be negligible. If they result in a loss of over 10% in complete-case analyses, the reasons of non-response are thoroughly investigated by asking the patients directly. When this is impractical, information is gathered from physicians, nurses, and any other available sources on a patient-to- patient basis. If the missing data generating mechanism is considered missing at random, the probability of response is estimated with logistic regression including the response indicator as the dependent variable and all its possibly relevant predictors as independent variables. The analyses are weighted by the inverse of the probability of response. If the missing data generating mechanism is deemed non-ignorable, their possible implications are assessed by sensitivity analyses.
For evaluation of secondary endpoints (clinical progression, prolonged time to onset of liver failure or reduce risk for complications measured by MELD and Child scores, worsening features at MRI, development of cirrhosis, onset of ascites, variceal bleeding, encephalopathy) we analyze time to each event separately. The end of follow-up is considered a right-censoring event, while the earliest among death, LT, and biliary malignancy, is considered a competing event. The endpoint of interest, the censoring event, and the competing event are analyzed by means of competing-risks regression models. Competing-risks regression posits a model for the sub-hazard function of a failure event of primary interest. The treatment group indicator is the only independent variable in the competing-risk regression models. We test the null hypothesis that the sub-hazard functions are equal in the two treatment groups by testing that the regression coefficient associated with the treatment indicator is equal to zero. The test is a two-sided, 0.05-level, Wald’s test. All the parts of the plan described above for the first primary aim also apply to this second aim.
Interim analysis
An interim analysis will be conducted with the first aim to ensure security aspects and secondly to evaluate if the study could stop earlier than intended due to benefit or futility. The interim analysis is performed when 50% of the patients (n=350) have fulfilled 5 years in the study. An independent statistician, blinded to exposure, will perform the statistical analysis. Primary outcomes and serious adverse events (SAEs) are evaluated. To keep the overall level of the test for differences between groups for the primary outcome at 0.05, we apply the Haybittle–Peto boundary level of 0.001 for stopping the trial at the interim analysis [26]. The trial is stopped for safety reasons if the difference between groups in SAE is significant at the 0.05 level.
Safety monitoring and assessment
Symptoms related to PSC or IBD are registered but not defined as potential adverse events (AE). AEs are registered at every visit in the electronic case report form using the Castor Electronic Data Capture platform. Only symptoms suspected being associated to the study medication and leading to a dose reduction or cessation will be registered as AE. If a patient develops myalgia or myopathy, a serum level of creatin kinase (CK) will be checked and the drug discontinued if the serum level of CK is 5-fold the baseline value. In the case of development of clinical rhabdomyolysis, the study medication will be discontinued. If a patient develops elevated liver function tests during follow-up the algorithm defined by Chalasani et al. will be used to manage and monitor these findings [27].
Serious Adverse Events (SAEs) or Suspected Unexpected Serious Adverse Reactions (SUSARs) are registered as soon as possible and sent to the monitoring center Clinical Trials Office, Karolinska University Hospital in line with good clinical practice (GCP).
Criteria for study termination
Study medication is terminated if the patient reaches a primary endpoint or after 5 years study participation. The patient has the right to terminate his/her participation in the study at any time without providing any reason. Data that has been collected until the termination will be used in the final analysis of the study. The responsible physician can exclude a patient from the study if any of the following occurs: a) pregnancy; b) serious adverse event that can be associated with the study medication; c) if a pause in study medication is longer than 3 months; d) if a medical indication for statin treatment or treatment with other lipid-lowering medication according to national guidelines has arisen.
Patient involvement
Patient involvement is important in clinical practice and research. Patient reported outcomes measures (PROMS) is to patients equally important to other secondary endpoints especially in a disease where effective treatment is lacking. To ensure patients´ perspectives when planning this study, we contacted the patient organization “PSC-Sverige”, and through this network invited patients and their relatives to a workshop at Karolinska University Hospital, Sweden. Upon request from the patients’ representatives a food frequency questionnaire FFQ (Meal-Q) was included, and life quality questionnaires were included as PROMS. Furthermore, a reference group of patient representatives have reviewed the patient information of this study.
Discussion
The aim of the current trial is to study whether treatment with simvastatin at a dose of 40 mg daily improves the prognosis in PSC. We also want to study the effect of statin therapy in PSC on cholestasis, symptoms, biomarkers for prognosis, immunological response, and other prognostic markers.
There is currently no treatment that slows disease progression or prevents death, LT or cancer development in PSC. There is therefore a great need to find effective medical therapy that improves prognosis. Statins have shown efficacy in other chronic liver diseases [9]. Both in decompensated cirrhosis and primary biliary cholangitis (PBC), statins were shown to be safe and to improve vascular function [24, 28]. The rational for a possible effect in cholestatic disease, in addition to the beneficial effect expected in all cirrhotic patients, is decreased levels of cholesterol both in serum and bile that may reduce inflammation, bile stone formation and ameliorated cholestasis [29]. Decreased cholesterol synthesis may result in decreased synthesis of toxic bile acids. We have tested the hypothesis that statins are effective in PSC in a Swedish registry study. It shows a halved risk of death, LT or cancer [8] which was used as measure of potential efficacy in the power calculation.
Statins have a favorable risk profile with few side effects and a very large number of people have been exposed to the drug without serious side effects. The potential effect of this treatment strategy is therefore considered to, by far, exceed the risks as PSC is a disease that lacks pharmacological treatment, is progressive and has a serious prognosis. The drug is also cheap. The chosen substance, simvastatin, is motivated by the fact that it is the most widely used statin in chronic liver disease and the most frequent in the registry study [8]. The side effect profile is favorable. The most common side effects are nasopharyngitis, allergic reactions, hyperglycemia, sleep disturbances, nightmares, headaches, blurred vision, abdominal problems (constipation, flatulence, dyspepsia, nausea, diarrhea), myalgia, abnormal liver function tests, and elevated serum CK. The dose of 40 mg daily may be considered medium and is motivated by the fact that we want to ensure that a treatment effect is not absent at the same time as we want to keep the dose within a reasonably low level to reduce dose-dependent side effects and the dose can be halved in case of pronounced side effects, based on the assessment of the treating physician, or at signs of liver failure [24].
There are specific challenges in this study. First, PSC is a rare and slowly progressive disease which makes enrollment of the required number of 700 patients a challenge. Our currently existing national liver research network for clinical studies in liver disease, SWEHEP, enables adequate inclusion. The broad population-based recruitment setting enables a high generalizability of the result given an adequate number of patients that are participating.
Many previous PSC studies were underpowered. If the effect size is less significant than the expected HR 0.5, also the present study might be underpowered to find a statistically significant difference in outcomes. Enrolment failure would further contribute to such a type II error. A false negative result would be clinically misleading and suggest that statins provide no or limited benefit. A robust surrogate marker would mitigate such a risk. The lack of markers for disease progression in PSC underscores the challenge of this study. To extenuate this risk and improve the possibilities for a clinical useful result, a systematic evaluation of progression of imaging features (MRI) will be done together with evaluation of development of symptoms and complications and quality of life measures (PROMS). This will hopefully add important information on simvastatin´s potential beneficial effect especially in PSC patients without advanced disease at baseline. Added value of this prospective large study is also that the placebo arm creates high quality natural history data that may serve as historical controls for future open labeled studies.
The double-blind study design is essential since it is well-known from cardiovascular medicine that the nocebo effect is important for development of side effects in statin treatment. Both physicians and patients contribute to the nocebo effect. A high rate of muscular and other symptoms attributed to statins are reported in observational studies and clinical practice, but not in randomized controlled trials [30].
Conclusion
PiSCATIN is the first long term randomized controlled trial in PSC since 2009 and is designed to assess the effect of simvastatin in prognosis of PSC. The composite endpoint of biliary malignancy, LT, variceal bleeding or death is clinically the most relevant and will give robust evidence of the efficacy of simvastatin in PSC.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study has received financial support from Swedish Research Council
Clinical trial registration: ClinicalTrials.gov with the identifier NCT04133792.
References
1. Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis - a comprehensive review. J Hepatol 2017;67:1298-1323.
2. Jepsen P, Gronbaek L, Vilstrup H. Worldwide Incidence of Autoimmune Liver Disease. Dig Dis 2015;33 Suppl 2:2-12.
3. Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013;58:2045-2055.
4. Lundberg Bave A, Bergquist A, Bottai M, Warnqvist A, von Seth E, Nordenvall C. Increased risk of cancer in patients with primary sclerosing cholangitis. Hepatol Int 2021.
5. Saffioti F, Gurusamy KS, Hawkins N, et al. Pharmacological interventions for primary sclerosing cholangitis: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3:CD011343.
6. Poropat G, Giljaca V, Stimac D, et al. Bile acids for primary sclerosing cholangitis. Cochrane Database Syst Rev 2011:CD003626.
7. Ponsioen CY, Lindor KD, Mehta R, et al. Design and Endpoints for Clinical Trials in Primary Sclerosing Cholangitis. Hepatology 2018;68:1174-1188.
8. Stokkeland K, Hoijer J, Bottai M, et al. Statin Use Is Associated With Improved Outcomes of Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol 2019;17:1860-1866 e1861.
9. Kim RG, Loomba R, Prokop LJ, et al. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2017;15:1521-1530 e1528.
10. Wijarnpreecha K, Aby ES, Ghoz H, et al. Statins and Risk of Cholangiocarcinoma: A Systematic Review and Meta- analysis. J Gastrointestin Liver Dis 2020;29:629-635.
11. Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 2009;50:808-814.
12. Olsson R, Boberg KM, de Muckadell OS, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology 2005;129:1464-1472.
13. Lindkvist B, Benito de Valle M, Gullberg B, et al. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology 2010;52:571-577.
14. Ponsioen CY, Chapman RW, Chazouilleres O, Hirschfield GM, Karlsen TH, Lohse AW, Pinzani M, et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: Review and results from an International PSC Study Group consensus process. Hepatology 2016;63:1357-1367.
15. Muir AJ, Levy C, Janssen HLA, et al. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease. Hepatology 2019;69:684-698.
16. Trivedi PJ, Muir AJ, Levy C, et al. Inter- and Intra-individual Variation, and Limited Prognostic Utility, of Serum Alkaline Phosphatase in a Trial of Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol 2020.
17. Vesterhus M, Hov JR, Holm A, et al. Enhanced liver fibrosis score predicts transplant-free survival in primary sclerosing cholangitis. Hepatology 2015;62:188-197.
18. Ruiz A, Lemoinne S, Carrat F, et al. Radiologic course of primary sclerosing cholangitis: assessment by three-dimensional magnetic resonance cholangiography and predictive features of progression. Hepatology 2014;59:242-250.
19. Lemoinne S, Cazzagon N, El Mouhadi S, et al. Simple Magnetic Resonance Scores Associate With Outcomes of Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol 2019;17:2785- 2792 e2783.
20. Grigoriadis A, Ringe KI, Andersson M, et al. Assessment of prognostic value and interreader agreement of ANALI scores in patients with primary sclerosing cholangitis. Eur J Radiol 2021;142:109884.
21. Pedersen TR, Tobert JA. Simvastatin: a review. Expert Opin Pharmacother 2004;5:2583-2596.
22. Abraldes JG, Albillos A, Banares R, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology 2009;136:1651-1658.
23. Abraldes JG, Villanueva C, Aracil C, et al. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology 2016;150:1160-1170 e1163.
24. Pose E, Napoleone L, Amin A, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol 2020;5:31-41.
25. Kaplan DE, Mehta R, Garcia-Tsao G, et al. SACRED: Effect of simvastatin on hepatic decompensation and death in subjects with high-risk compensated cirrhosis: Statins and Cirrhosis: Reducing Events of Decompensation. Contemp Clin Trials 2021;104:106367.
26. Pocock SJ. When (not) to stop a clinical trial for benefit. JAMA 2005;294:2228- 2230.
27. Chalasani N, Regev A. Drug-Induced Liver Injury in Patients With Preexisting Chronic Liver Disease in Drug Development: How to Identify and Manage? Gastroenterology 2016;151:1046-1051.
28. Stojakovic T, Claudel T, Putz-Bankuti C, et al. Low-dose atorvastatin improves dyslipidemia and vascular function in patients with primary biliary cirrhosis after one year of treatment. Atherosclerosis 2010;209:178-183.
29. Schierwagen R, Uschner FE, Magdaleno F, et al. Rationale for the use of statins in liver disease. Am J Physiol Gastrointest Liver Physiol 2017;312:G407- G412.
30. Tobert JA, Newman CB. The nocebo effect in the context of statin intolerance. J Clin Lipidol 2016;10:739-747.
Received: March 02, 2022;
Accepted: March 18, 2022;
Published: March 20, 2022.
To cite this article : Bergquist A, Marschall HU, Nilsson E, et al. Long Term Effect of Simvastatin in Primary Sclerosing Cholangitits: A Placebo-Controlled, Double-Blind, Multicenter Phase III Study (Piscatin). British Journal of Gastroenterology. 2022; 4(1): 235- 241. doi: 10.31488/bjg.1000128.
© Bergquist A, et al. 2022.
