Case Report / Open Access
DOI:10.31488/bjg.1000133
Persistence of Gastric Metaplasia of the Terminal Ileum after Post-transplant Cytomegalovirus Ileitis and Graft-versus-Host Disease
Alexander H. Yang, MD, MS*1, Theo Heller, MD2, Robert D. Shamburek, MD3, Sheila Kumar, MD, MS1, Chyi-chia Lee, MD4, Noa G. Holtzman, MD5, Steven Z. Pavletic, MD5
1. Digestive Diseases Branch, National Institute of Diabetes, Digestive, and Kidney Diseases. National Institutes of Health, Bethesda, Maryland, USA
2. Translational Hepatology Section, Liver Diseases Branch, National Institute of Diabetes, Digestive and Kidney Diseases. National Institutes of Health, Bethesda, Maryland, USA
3. National Heart Lung and Blood Institute, National Institutes of Health, Bethesda, Maryland, USA
4. Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
5. Immune Deficiency Cellular Therapy Program, National Cancer Institute, Center for Cancer Research, National Institutes of
*Corresponding author: Alexander H. Yang, Digestive Diseases Branch, National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health, 10 Center Drive, Room 52740, Bethesda, Maryland 20892, USA
Abstract
Gastric metaplasia is the change of native mucosa to gastric tissue outside of the stomach, a rare entity that is not well described in the literature and for which the clinical implications and management is not well understood. Herein, we describe a case of a patient with relapsed/refractory stage IVB Hodgkin lymphoma who underwent an allogeneic hematopoietic stem cell transplantation (HSCT) that was complicated by graft-versus-host disease (GVHD) requiring multiple lines of systemic immunosuppression as well as infections including cytomegalovirus (CMV). Endoscopy done to explore etiology of persistent gastrointestinal symptoms showed evidence of GVHD and CMV ileitis as well as gastric metaplasia in the terminal ileum. GVHD and CMV were both treated to resolution. Given the unknown implications of the metaplasia he underwent repeat colonoscopy for monitoring and on Day +859 post-HSCT still showed persistent gastric metaplasia with normal findings on endoscopy. The significance and natural history of gastric metaplasia in the terminal ileum is not well understood. We hypothesize that in this patient it is a reaction to tissue injury from GVHD as well as CMV ileitis. Close monitoring and repeat colonoscopy should be considered to ensure no progression to dysplasia, especially in the immunosuppressed post-HSCT patient.
Keywords: Gastric metaplasia, hematopoietic stem cell transplantation, graft-versus-host disease, cmv, ileitis
Introduction
Gastric metaplasia is the transformation of original gastrointestinal (GI) mucosa to gastric tissue outside the stomach, and has been rarely described in the literature, including in duodenum in the setting of inflammatory bowel disease, foveolar gastric metaplasia as duodenal polyp and even in the endometrium [1-3]. The pathogenesis and implications of this entity are not well understood, compared to other forms of metaplasia in the GI tract, such as Barrett’s esophagus. Herein, we report a case of gastric metaplasia of the terminal ileum (TI) in a post-allogeneic hematopoietic stem cell transplantation (HSCT) patient with longitudinal follow up [4].
Case
A 37-year-old Hispanic male with relapsed/refractory stage IVB Hodgkin lymphoma underwent a reduced intensity matched unrelated donor peripheral blood HSCT that was complicated by grade IV late acute graft-versus-host disease (GVHD) involving the skin, GI tract, and liver. His GVHD did not respond to initial steroids and required a prolonged course of second-line systemic immunosuppressive therapies (IST) including mycophenolate mofetil, ruxolitinib, extracorporeal photopheresis, and antitrypsin protease inhibitor to achieve remission. His course was further complicated by several infections, including cytomegalovirus (CMV) reactivation in the blood requiring preemptive therapy with intravenous ganciclovir. Due to persistent diarrhea and abdominal pain with fluctuating GVHD activity and CMV viremia, he underwent multiple endoscopies for further investigation. Colonoscopy on day (D)+169 post-HSCT grossly showed inflammation and friable ulceration in the TI (Figure 1A), and biopsy was positive for CMV PCR and stains as well as frequent apoptotic bodies consistent with concurrent CMV ileitis and GVHD involvement (Figure 2A-D). Repeat colonoscopy on D+250 showed normal colon, and multiple ulcers in the TI (Figure 1B) with biopsy demonstrating presence of Paneth cells within gastric glandular mucosa, favoring gastric metaplasia rather than gastric heterotopia with features suggestive of injury associated response as well as CMV (Figure 3E-F). After multiple lines of IST and a prolonged hospital stay, the CMV viremia was cleared with intermittent recurrent reactivation, and gut GVHD achieved a complete remission with symptom resolution. Patient was discharged on prednisone and tacrolimus for continued slow taper.
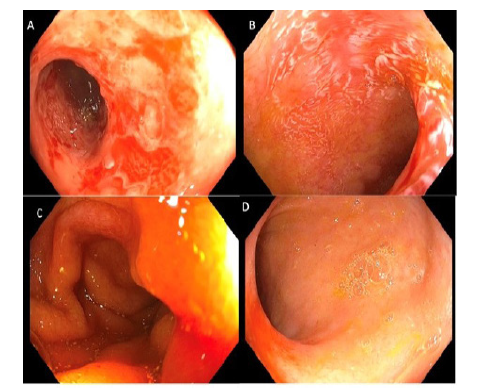
Figure 1.Endoscopy Findings
A. Day +169: terminal ileitis
B. Day +250: terminal ileum with multiple ulcers with contact
bleeding
C. Day +451: Normal terminal ileum
D. Day +859: Normal terminal ileum
Repeat colonoscopy on D+471 and +859 showed normal colon and TI (Figure 1C-D). Biopsy on D+471 showed ileal mucosa with prominent reactive germinal centers, consistent with persistence of gastric metaplasia in the TI but with signs of healing and improvement, and D+859 showed ileitis and again gastric foveolar glands with interstitial infiltrated with lymphocytes and plasma cells, surfaced by gastric epithelium (Figure 3G-J).
Discussion
Gastric metaplasia is a rare finding of gastric tissue outside of the stomach and is even less commonly seen in the ileum. The pathogenesis is not well understood and thought to be secondary to inflammation such as inflammatory bowel disease [1]. This entity can be confused with gastric heterotopia which is thought to be congenital. No specific treatment is advised or required, and the implications, including neoplastic potential, are not described. In our patient, we noted development of gastric metaplasia that persisted on follow up. The inflammatory process that causes damage to the gut epithelium from both acute GVHD and CMV ileitis could be a predisposition for development of metaplasia. Whether or not this condition resolves with long term follow up is unknown, especially as many of these post-HSCT patients are on prolonged IST, which may impair tissue repair and increase risk of secondary malignancies [4]. The natural history of this rare entity is unclear, therefore, close monitoring to ensure no progression to dysplasia is important, with special attention in post-HSCT patients whose course is complicated by GVHD and CMV.
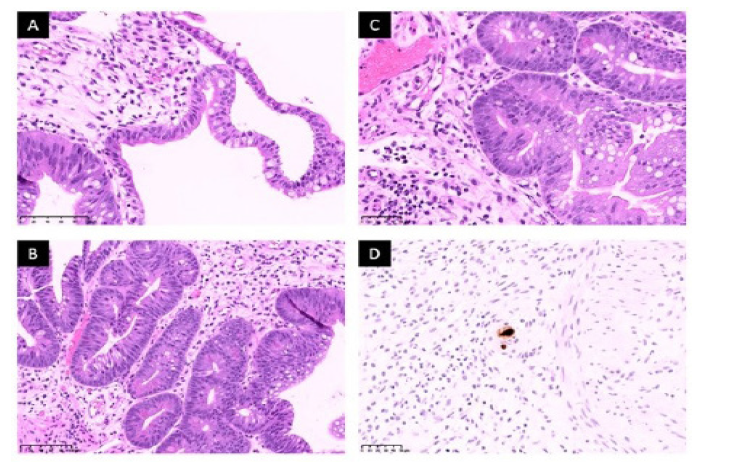
Figure 2.Histology
A. (Day +169) Initial biopsy of the terminal ileum revealed foci of goblet cells, consistent with intestinal mucosa. There is granulation
tissue and focal mild chronic inflammation. The adjacent glandular mucosa show metaplastic changes with reactive atypia. B. (Day +169)
There is gastric glandular metaplasia and residual Paneth cells. C. (Day +169) Apoptotic bodies are present along the base of glands, consistent
with involvement by GVHD. D. (Day +169) CMV immunohistochemistry confirmed the presence of viral inclusions, consistent
with CMV ileitis.
Histology methods: Biopsy specimen are fixed in 10% neutral buffered formalin and routinely process for H&E sections. Immunohistochemistry
for CMV was performed using automated stainer per NCI Laboratory of Pathology protocols.
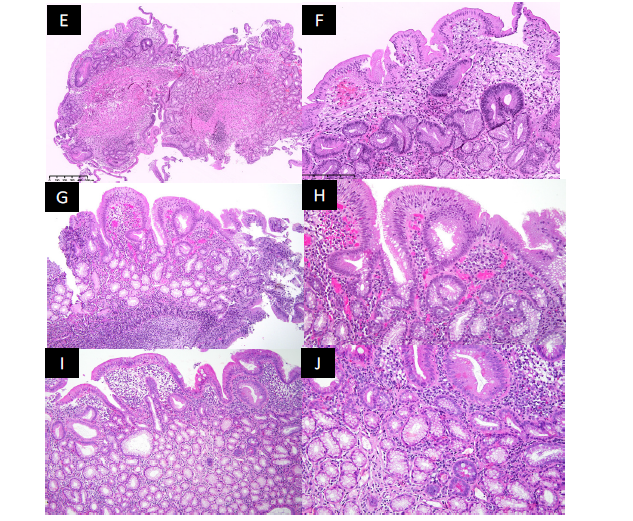
Figure 3.Histology. E. (Day +250) Subsequent biopsy of the terminal ileum revealed predominantly gastric foveolar glands with gastric mucosal epithelium and foci of lymphocytic infiltrate. There is complete absence of intestinal villi, intestinal crypts, and Peyer’s patches in this biopsy specimen. F. (Day+250) Higher magnification view of the subsequent terminal ileum biopsy revealed complete absence of goblet cells and Paneth cells. The gastric foveolar glands show architectural disorder with foci of reactive atypia, suggestive of reparative changes. There is also background of granulation tissue and mild chronic inflammation. G. Low magnification view of the biopsy from terminal ileum revealed mostly gastric foveolar glands, surfaced by gastric epithelium. There is a reactive lymphoid follicle. (Day +471) 10x . H. Higher magnification view of the biopsy from terminal ileum showed mostly gastric foveolar glands with interstitial infiltrate with lymphocytes and plasma cells. (Day +471) 20x. I. Low magnification view of the biopsy from terminal ileum revealed mostly gastric foveolar glands (some are dilated and irregularly shaped), surfaced by gastric epithelium. There is villous architecture and lymphoplasmacytic infiltrate. (Day +859) 10x
Guarantor of the Article
Alexander H. Yang and Noa Holtzman
Author Contributions
All authors contributed to the writing and editing of the manuscript and approve the final version. AHY: conception, collection of data, writing of manuscript, approval of final draft. CCL: Conception, collection of data, providing biopsy images, approval of final draft. TH, RDS, SK: Editing and writing review of the manuscript. NGH, SZP: Conception, writing of manuscript, senior supervision, approval of final draft
Financial Support
This work was supported by funding from the Intramural Research Program, National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Potential Competing interest
The authors declare no conflict of interest.
Statement of Consent
We confirm that the patient has provided informed consent to this manuscript.
References
1. Ikezono G, Yao K, Imamura K, et al. Gastric metaplasia of the duodenal mucosa in Crohn's disease: novel histological and endoscopic findings. Endosc Int Open. 2021 Feb;9(2):E181-E189.
2. Toussaint C, Libbrecht L, Dano H, et al. Endoscopic features, pathological correlates and possible origin of foveolar gastric metaplasia presenting as a duodenal polyp. Acta Gastroenterol Belg. 2019 Apr-Jun;82(2):257-260.
3. Wong RW, Talia KL, McCluggage WG. Endometrial Gastric-type Carcinoma: An Aggressive and Morphologically Heterogenous New Histotype Arising From Gastric Metaplasia of the Endometrium. Am J Surg Pathol. 2020 Dec;44(12):1736-1737.
4. Lum SH, Slatter MA. Malignancy post-hematopoietic stem cell transplant in patients with primary immunodeficiency. Expert Rev Clin Immunol. 2020 May;16(5):493-511.
Received: May 31, 2022;
Accepted: June 20, 2022;
Published: June 22, 2022.
To cite this article : Yang AH, Heller T, Shamburek RD, et al. Persistence of Gastric Metaplasia of the Terminal Ileum after Post-transplant Cytomegalovirus Ileitis and Graft-versus-Host Disease. British Journal of Gastroenterology. 2022; 4(2): 261-263. doi: 10.31488/bjg.1000133.
© Yang AH, et al. 2022.
