Research article / Open Access
DOI:10.31488/bjg.1000143
Pre-Diabetes Reduction from ALCAT Food Allergen Testing: A Controlled, Pilot Study
F. Buck Willis PhD, MDc, FACSM *1,4, Parminder S. Chahal MD2, Bronson Flint MS4,1, Patch Adams, MD3, Andrew Merry MD, PhD1
1. International University of Health Sciences (IUHS), School of Medicine, Basseterre, Saint Kitts; Nevis, USA
2. Integrity Internal Medicine, Peoria AZ, USA
3. Gesundheit Institute, Urbana IL, USA
4. Galveston Clinical Research, Galveston TX USA
*Corresponding author: Dr FB Willis, Galveston Clinical Research, P.O Box 1267 Basseterre, St Kitts, Nevis, 6341 Stewart Rd 115, Galveston TX 77551, USA
Abstract
Introduction: Type 2 Diabetes (T2D) effects over 500 million people, worldwide, and the cost of this disease is estimated to exceeded $997 billion US dollars. T2D is comorbid with obesity and hypertension, and the purpose of this 6-month case/control study was to determine if food allergy elimination is effective in reducing the HbA1c and BMI values in Pre-diabetic (PDM) patents in the USA and Caribbean. Methods: Twenty-four PDM subjects (with obesity and hypertension) enrolled in this case/control pilot study. All subjects received nutritional counseling and aerobic exercise instructions which is the initial standard of care for PDM. Control (CON) subjects received no further treatment. Experimental (EXP) subjects also had ALCAT leukocyte reaction testing to identify sub-acute food allergens (N=250 foods). Food Allergens were eliminated for the 6-month study duration. The dependent variables in this study were changes in HbA1c and BMI values. The independent variables included changes in fat mass, blood pressure, and waist circumference. Differences were calculated using a repeated measures ANOVA. Results: After 180 days, the data revealed that EXP subjects showed reductions in A1c and BMI (-0.7 HbA1c, -1.1 BMI), while CON showed increased values (+0.5 HbA1c, +0.5 BMI), (P < 0.0001). EXP also showed significant changes in Blood pressure (-25% systolic, -20% diastolic), Fat Mass (-18 pounds), and waist circumference (-2”). There were not significant changes in these variables for CON subjects.
Keywords: leukocyte reaction testing, aerobic-surge exercises, obesity reduction
Introduction
Globally, 537 million adults are living with diabetes, and that incidence is expected to increase by 20% to 643 million in 2030. Additionally, an estimated 350 million children and adolescents are now overweight with undiagnosed PDM [1-5] PDM plus Type 2 Diabetes (T2D) effects over 50 million people in the USA. In the Caribbean and Central America countries, T2D incidence has grown 30% in the past ten years [4-14] and is expected to reach 50% by 2045.
In the Caribbean, diabetes is 4th leading cause of death reaching almost 14% of all mortalities, and Hypertension (HTN) with heart disease are often comorbid with T2D and PDM [4-6]. Eighty percent of the world’s diabetes population reside in low to moderate income countries [46,12-15]. The current global cost of treating diabetes exceeds $997 billion US dollars which has shown a 316% increase over the last 15 years [1-10].
Weight loss has been shown to reduce and slow the evolution of PDM to T2D, however research has not shown the effect of eliminating sub-acute food allergies, which may be a contributing cause of PDM, as it has been shown in obesity [7-11]. Numerous studies have examined treating the symptoms of T2D and obesity with diet, nutrition [5-15] and therapeutic exercise [7-11,20], however only the newest studies have examined food allergen elimination for obesity and pre-diabetes reduction [7-11].
There is a lack of clinical research examining the underlying causes of PDM and the obesity epidemic, but sub-acute food allergy elimination has long been named as a contributing factor of many diseases from migraine headaches to irritable bowel syndrome [7-11,15,16,23-29].
A recent 12-month study examined treating obesity with food allergen elimination and with using the brief, repeated Aerobic-surge exercise routine [11]. That multi-centre, case/control study (N=94, ages 18-76) compared four subject categories: 1. Food Allergen elimination with brief Aerobic-surge exercises, (5/day); 2. Food Allergen elimination alone; 3. Aerobic-surge exercises alone (5/day); and 4. Control.
All subjects received nutritional assessment, counseling, and guidance to avoid problematic foods with ingredients such as TBHQ, high fructose corn syrup, etc. The first two groups of subjects had ALCAT Leukocyte Reaction testing for food allergies which examined reactions from 237 foods (Cell Science Systems Testing, Deerfield Beach, Florida, USA). Foods that showed a significant reaction to this test were eliminated from their diet (mean 36 foods per subject) [7-11,20, 22].
The results of that study by Willis et al. were both significant and meaningful. All therapeutic groups displayed significant changes in weight, BMI, and girth measurements, however the greatest change was for Group I which employed food allergen elimination and Aerobic-surge exercises (p < 0.0001). That group displayed the greatest mean changes in Weight -32.2lbs (14.6kg), BMI -4.6, and Waist circumference -4.3” (10.9cm) [11].
The correlations between PDM, T2D, and obesity are substantial [1-32]. Recently the US CDC reported that the prevalence of allergies, obesity and pre-diabetes were significant, because 73% of Americans have allergies and over 80% of Americans with PDM or T2M patients are also obese, (This data came from a recent survey of 28,429 patients) [31]. The purpose of this 6-month study was to determine if food allergy elimination is effective in reducing the HbA1c and BMI values in PDM patents.
Methods
Diagnosis of PDM was accomplished with Hemoglobin A1c testing at private medical clinics with values of 5.7-6.4. The A1c test values above 6.5% yielded diagnosis of T2D and these candidates were not included in this study. Diagnosis of obesity is defined with the Body Mass Index (BMI) scale and readings > 30 are obese.
The BMI formula is;
BMI= Body Weight (kg) ÷ Height (m2)
Twenty-four (N=24) subjects enrolled in this case/control trial who were newly diagnosed with PDM plus Obesity, and these subjects were recruited from four private, primary care clinics (Peoria AZ, Clear Lake TX, and Galveston TX, and Basseterre St Kitts in the Caribbean). The mean subject age was 45, (range 18-82 years), with 2 males, and subjects of Caucasian, Hispanic, and African ethnicities. After a briefing on the procedures, risk, and benefits, each subject signed the informed consent as required by the Galveston Clinical Research IRB (#01.2021).
Then all subjects had nutritional assessment completed with 7-day diet tracking. This was used for nutritional assessment and dietary recommendation such as eliminating foods with High Fructose Corn Syrup, TBHQ, and other detrimental preservatives [11,17-19]. Plus, subjects were taught how to eat smaller, more frequent “feedings” [11,17]. For example, if one’s total daily volume of food is one liter, it would be divided into five “feedings” compared to the traditional three meals per day of the same total daily volume.
All subjects were also taught how to perform the Aerobic-surge exercise protocols which started with calculation of each subject’s age-specific, estimated Aerobic Threshold (AT). The Aerobic-surge protocol was shown previously beneficial [11,20], and the AT formula is:
(220 – age) x 75% = AT [7-11,20].
Subjects were instructed to perform five of the 2-minute Aerobic-surges each day and compliance was measured with daily electronic reports from all subjects. Example exercises were shown in the book “Food is the Cure for the Overweight Disease” [15] and this book was given to all subjects.
All subjects reported their compliance with weekly food diaries and their “daily count” of the total number Aerobic-surges performed of feedings were sent to the Dr. Willis daily, (such as “5/5” or “3/3”).
After the nutritional assessment and Aerobic-surge exercise training, subjects chose whether they would like to be treated with diet and exercise alone, as control subjects (CON) which is the initial, standard of care for PDM, or if they would also like to be also treated with sub-acute food allergy testing and elimination using the ALCAT Leukocyte Reaction test. The Experimental (EXP) subjects (N=12) who chose the leukocyte reaction testing had three vials of blood drawn once for analysis by the Cell Science System labs (ALCAT, Cell Science Systems Labs, Deerfield Beach, FL, USA).
Results of the ALCAT test were discussed between the PI and subjects (figure 1) and subjects were told to eliminate the allergens for the duration of this study and they were given dietary suggestions taken from the foods which showed “No significant reaction.” For example, if a person showed a significant allergic reaction to a water chestnut, the PI would suggest a substitute like almonds or cashew nuts. All food allergens were eliminated for the full 6-month duration of this study.
Figure 1:Sample ALCAT test report
Results
Differences were calculated using a repeated measures ANOVA. Testing after 180 days, showed a significant change in HbA1c and BMI for the EXP subjects (-0.7 A1c and -1.1 BMI) and in comparison, the CON subjects’ HbA1c actually rose by (+0.5 A1c and +0.5 BMI), (See Figure 2). There was a significant difference in the dependent variables of HbA1c and BMI (P< 0.0001, SD=0.343, F-ratio 85.6.) and there were also significant changes for EXP subjects in Blood Pressure, (-3.8 kg Fat Mass, -25% systolic BP, and -20% diastolic BP). Four subjects disenrolled: one due to pregnancy, one due to anew cardiac diagnosis, and two due to loss of interest, (2 CON and 2 EXP lost).
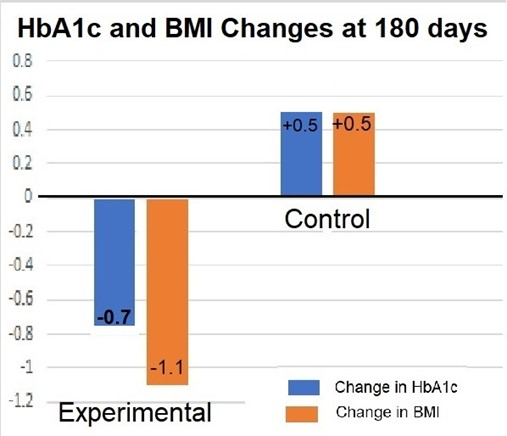
Figure 2:Results
Discussion
These findings concurred with previous studies of food allergen elimination for obesity reduction by Lewis et al. They tested 120 overweight subjects for IgG food allergens and foods that had a positive reaction were removed for experimental subjects’ diets for 90 days. These subjects saw significant changes in weight and waist/hip circumferences: Weight – 5kg and Waist circumference -5.4cm, (P <0.01) [23]. One limitation of that study by Lewis et al. was that they only tested for IgG reactions.
Whereas the ALCAT Leukocyte reaction testing examines changes in the size and number of the total population of circulating white blood cells in whole blood, following individual, ex vivo challenges with standardized food extracts, thus providing significantly more information about how the total immune system responds to the particular test substance in a non-mechanism dependent manner.
While food allergy testing and elimination have been studied previously for reduction of migraine headaches and intestinal disorders [25-29], the research has not been extensive in determining the efficacy of food allergy elimination in management of Obesity or PDM reduction. However, the food allergen elimination studies have shown ideal safety with zero adverse events [7-11,27,28].
Food is touted as both the cause and the cure for PDM and obesity. Processed foods and chemicals such as aspartame have been shown to be contributing causes, while natural foods have been shown to help treat these diseases [6-15]. It has also been concluded that an elevated BMI is both a global health risk and biomarker for specific cardiovascular diseases. While Obesity is a continually growing epidemic, the cases of Obesity and Pre-Diabetes grew at an even greater rate during the COVID-19 pandemic [30].
Conclusion
T2D and obesity have a rapidly growing incidence in the Caribbean and publications have shown that it could be the highest region for this comorbidity [2,4,32]. The purpose of this 6-month, controlled pilot study was to determine if food allergy elimination is effective in reducing the HbA1c values in Pre-diabetic (PDM) patents.
The results of this controlled pilot study showed significant changes and differences in HbA1c and BMI as well as the independent variables (p < 0.0001). Next a randomized, controlled trial should be conducted to show efficacy of using food allergen elimination and the Aerobic-surge exercise protocol so the growth in PDM and obesity can be reversed.
Conflict of Interest
No conflict of interest exists between the authors and the sponsoring Cell Science Systems company. Cell Science Systems donated the ALCAT testing as well as publications fees and travel for research presentations.
References
1. Diabetes UK.2024 Aug.
2. American Diabetes Association .2024 Aug.
3. US Center for Disease Control, Diabetes .2024 July.
4. International Diabetes Federation (IDF), Diabetes Atlas 2021. 2024 July.
5. NIH, Pre-Diabetes Atlas . 2024 July.
6. Guariguata L, Garcia L, Sobers N, Ferguson TS, Woodcock J, Samuels TA, et al. Exploring ways to respond to rising obesity and diabetes in the Caribbean using a system dynamics model. PLOS Glob Public Health. 2022 May 19;2(5):
7. Willis FB, Flint B, Merry A, Adams P, Chahal P, Malik F, et al. Pre-Diabetes Reduction from Food Allergen Elimination: a Controlled, Pilot Study. 84th America Diabetes Association, Scientific Conference.
8. Willis FB, Parenti S, Adams HP. Prediabetes Reduction from Food Allergen Elimination. Med Clin Case Rep. 2023; 3(1): 1-3.
9. Willis FB, Arogundade R, Teimouri E, Maule N. Adolescent Pre-diabetes Reduction from Food Allergen Elimination: a Case Report. British Journal of Gastroenterology. 2022;4(2): 254-256.
10. Willis FB, Dawoud S, Flint B, Chahal PS. Diabetes and Obesity Reduction from Food Allergen Elimination: A Two-Year Geriatric Case Report. Medical Clinical Case Reports; 2023:3(1)1-2.
11. Willis FB, Shanmugam R, Southerland JH, Mouton CP. Food Allergen Elimination for Obesity Reduction; a Longitudinal, Case-Control Trial. British Journal of Gastroenterology. 2020; 2(4): 199-203.
12. Butt MD, Ong SC, Wahab MU, Rasool MF, Saleem F, Hashmi A, et al. Cost of Illness Analysis of Type 2 Diabetes Mellitus: The Findings from a Lower-Middle Income Country. Int J Environ Res Public Health. 2022 Oct 2;19(19):12611.
13. Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process: a position statement of the World Obesity Federation. Obes Rev. 2017;18:715-723.
14. Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a disease: the Obesity Society 2018 position statement. Obesity (Silver Spring). 2019;27:7-9.
15. Willis FB. Food is the Cure for the Overweight Disease. Galveston Press, Amazon Kidle 2020.
16. Willis FB, Shanmugam R, Curran, SA. Food Allergen Eliminations and Combined Protocols for Obesity Reduction: a Preliminary Comparison Study. Food Sci Nutrition Res. 2018; 1(1):1-3.
17. Iwao S, Mori K, Sato Y. Effects of meal frequency on body composition during weight control in boxers. Scand J Med Sci Sports.1996; 6: 265-72.
18. Nardocci M, Leclerc BS, Louzada ML, Monteiro CA, Batal M, Moubarac JC, et al. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health. 2019 Feb;110(1):4-14.
19. Willis FB, Brackman SM. Food is the Cause and the Cure for the Obesity Epidemic. Medical Clinical Research. 2020:5(1):1-3.
20. Willis FB, Curran S. Brief, Aerobic-surge Exercises for Effective Weight Loss: a Randomized, Controlled Trial. Med Clin Res.2019; 4(11):1-5.
21. Echouffo-Tcheugui JB, Perreault L, Ji L, Dagogo-Jack S. Diagnosis and Management of Prediabetes: A Review. JAMA. 2023 Apr 11;329(14):1206-1216.
22. Sjjie T, Hainai Y, Fengying Y, Jianxiong W. High intensity interval exercise training in overweight young women. J Sports Med Phys Fitness. 2012 Jun;52(3):255-62.
23. Lewis JE, Woolger JM, Melillo A. Eliminating immunologically-reactive foods from the diet and its effect on body composition and quality of life in overweight persons. J Obes Weig loss Ther 2012: 2(1):1-6.
24. British Allergy Foundation, “Food Intolerance and Sensitivity,”.
25. Alpay K, Ertaş M, Orhan EK, Üstay DK, Lieners C, Baykan B, et al. Diet restriction in migraine, based on IgG against foods: A clinical double-blind, randomised, cross-over trial. Cephalalgia. 2010;30(7):829-837.
26. Palmer DJ, Huang RC, Craig JM, Prescott SL. Nutritional influences on epigenetic programming: asthma, allergy, and obesity. Immunol Allergy Clin North Am. 2014 Nov;34(4):825-37.
27. Arroyave Hernández CM, Echavarría Pinto M, Hernández Montiel HL. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex. 2007: Sep-Oct;54(5):162-8.
28. Ali A, Weiss TR, McKee D, Scherban A, Khan S, Fields MR, et al. Efficacy of individualised diets in patients with irritable bowel syndrome: a randomised controlled trial. BMJ Open Gastroenterol, 2017 Sep 20;4(1):e000164.
29. Atkinson W. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53(10):1459-1464.
30. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023 Jan 20;30(1):11-18.
31. Sun J, Liu Z, Zhang Z, Zeng Z, Kang W. The Correlation of Prediabetes and Type 2 Diabetes with Adiposity in Adults. Front Nutr. 2022 Apr 11;9:818263.
32. Bennett NR, Francis DK, Ferguson TS, Hennis AJ, Wilks RJ, Harris EN, et al. Caribbean Alliance for Health Disparities Research Group (USCAHDR). Disparities in diabetes mellitus among Caribbean populations: a scoping review. Int J Equity Health. 2015 Feb 25;14:23.
Received: February 02, 2024;
Accepted: February 26, 2024;
Published: February 29, 2024.
To cite this article : Willis FB, Chahal PS, Flint B, Adams P, Merry A. Pre-Diabetes Reduction from ALCAT Food Allergen Testing: A Controlled, Pilot Study. British Journal of Gastroenterology. 2025; 7(1): 312-317. doi: 10.31488/bjg.1000143.
© The Author(s) 2025. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
