Case report / Open Access
DOI:10.31488/bjg.1000138
The Diagnosis and Management of a patient with Idiopathic Encapsulating Sclerosing Peritonitis
Katie Nightingale*1, Linda Birtles2 , Malcolm Greenwood-Morgan2 , Aviram Nissan3 , David van Dellen2,4, Zia Moinuddin2,4, Titus Augustine2,4
1. Core Surgical Trainee: Manchester University Foundation Trust, UK
2. Manchester Royal Infirmary; Manchester University Foundation Trust, Oxford Road, Manchester M13 9WL: A UK national referral centre for Surgery for Encapsulating Peritoneal Sclerosis
3. Department of General and Oncological Surgery -Surgery C, Chaim Sheba Medical Centre, Tel Hashomer, Affiliated to Sackler School of Medicine, Tel Aviv, Israel
4. Faculty of Biology Medicine and Health, Division of Diabetes, Endocrinology and Gastroenterology, Manchester Academic Health Science Centre, University of Manchester, Manchester UK
*Corresponding author: Katie Nightingale Core Surgical Trainee: Manchester University Foundation Trust, UK
Abstract
Idiopathic or Primary Encapsulating peritoneal sclerosis (EPS) is a rare condition characterised by the formation of a fibrocollagenous membrane encasing the peritoneum and leading to bowel obstruction without any underlying local or systemic pathology. This entity has mainly been reported in East Asia and the exact pathophysiological mechanisms are speculative. Primary EPS is different from the more commonly known condition of secondary encapsulating peritoneal sclerosis associated with peritoneal dialysis or other systemic conditions. Once diagnosed, further dilemmas arise with planning definite management and surgical intervention. We present a case report of a 58- year-old, previously fit and well caucasian male patient with idiopathic encapsulating sclerosing peritonitis who presented with abdominal symptoms and bowel obstruction. The patient's clinical presentation, the diagnostic processes, definitive surgical management and outcomes are discussed. This case highlights and reviews this rare condition and management from the perspective of an experienced international referral centre for the surgical treatment of encapsulating peritoneal sclerosis.
Keywords: Idiopathic encapsulating peritoneal sclerosis, bowel obstruction, peritoneal encapsulation, abdominal cocoon syndrome, sclerotising peritonitis
Introduction
Encapsulating peritoneal sclerosis (EPS) is an infrequent and debilitating condition characterised by the formation of a fibrocollagenous membrane in the peritoneal cavity [1]. In the most advanced cases, the membrane can also be calcified. The term EPS is descriptive of the underlying pathophysiological process of this condition. The thickening (sclerosis) of the peritoneum, both visceral and parietal results in the formation of a pathognomonic cocoon (encapsulation) of part or all of the bowel, leading to recurrent episodes of partial or complete obstruction of the gut, mainly the small intestine. EPS can be categorised into two types: primary (idiopathic) and secondary, depending on whether a specific trigger for the inflammatory process can be identified [2]. Secondary EPS, is most commonly caused by long-term peritoneal dialysis and forms the largest number of cases described in literature. Other secondary causes include peritoneal infection, medications and inflammatory disorders luteinizing thecomas, and following transplantation [3-6].
Clinical presentation is varied and non-specific leading to delays in diagnosis. The most common presenting symptoms include malnourishment, abdominal pain, abdominal distension and vomiting. Many patients, despite the insidious nature of the disease, present with acute presentations such as acute small bowel obstruction or perforation due to complications of EPS arising prior to diagnosis [7].
Diagnostic methods for EPS typically involve computed tomography (CT) scans. Findings include a highly enhancing and calcified peritoneal lining and dilation and often adherence of small bowel loops with ascites. Most patients also undergo a diagnostic laparoscopy accompanied by peritoneal biopsy and the combination of clinical picture, CT imaging and surgical findings together the diagnosis is confirmed. Figure 1 (adapted from Danford et al.) shows a proposed flow diagram on the diagnosis of EPS.
Histopathological examination plays a crucial role in confirming the diagnosis, and the specific glycoprotein,Podoplanin, which binds to inflammatory cytokines, aids in distinguishing EPS from other conditions such as peritoneal sclerosis and peritonitis [8].
Once EPS is diagnosed, it is important to address the underlying condition. For EPS due to peritoneal dialysis, transitioning to haemodialysis is necessary. If it is related to medications they should be discontinued, and any underlying inflammatory conditions should be treated. However, even with the removal of the triggering factors, the abdominal symptoms often persist due to the fibrotic nature of the cocoon causing the obstructive picture. The approach to management varies depending on the degree and severity of clinical presentation and entails a combined medical and surgical strategy. There is evidence suggesting the use of immunosuppressive medications like corticosteroids and antifibrotic medications such as Tamoxifen have a role in the treatment of EPS and therefore steroids and Tamoxifen are typically administered when EPS is recognised and there are low grade symptoms without frank bowel obstruction [9]. Good nutrition is crucial for patients with EPS, and total parenteral nutrition (TPN) is indicated once enteral feeding is poorly tolerated in these cases and prior to definitive surgical intervention [10].
Surgical intervention is recommended when conservative therapies have failed [1]. Surgery is best carried out as a semi-elective procedure as emergency surgery leads to significant surgical morbidity and mortality. Surgery entails careful enterolysis, which involves careful dissection and removal of the thick fibrotic sac, leading to relief of bowel obstruction and complete recovery [6]. Emergency surgery is usually aimed at managing complications associated with EPS, such as acute obstruction, perforated or ischemic bowel or haemoperitoneum. While curative treatment is preferable, emergency intervention is technically challenging, time-consuming, and carries a high risk of enteric fistulae [6].
The overall mortality rate for EPS has been quoted between 35-50% [11]. One-year mortality rates in peritoneal dialysis patients with EPS are reported to be 50% [2]. Ultimately, early diagnosis and planned treatment gives the best outcomes, whether it be primary or secondary EPS.
Case Description
A 58-year-old male patient was admitted locally due to a onemonth history of poor appetite, abdominal pain, and abdominal distension. Prior to this, the patient had been in good health and had no previous medical or surgical conditions.
Upon admission, an initial CT scan revealed dilated loops of duodenum and proximal jejunum, along with collapsed ileal loops. There were also small amounts of cloudy free fluid surrounding the loops of small intestine, forming a sac-like structure.
To relieve the patient's symptoms, a nasogastric tube was inserted, and it initially drained a large volume of bile content.
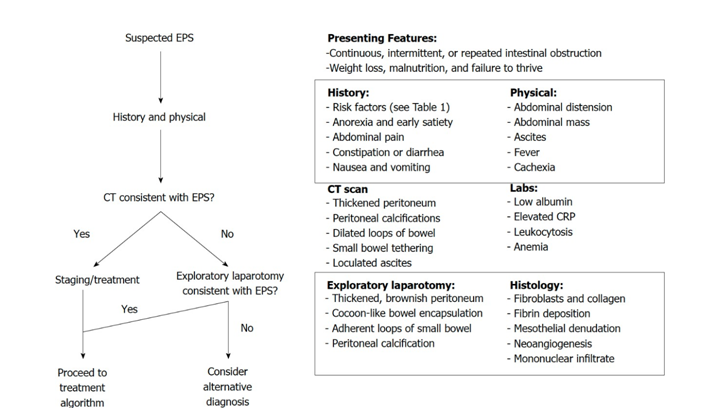
Figure 1:Proposed algorithm for the diagnosis of EPS. Adapted from Danford et al.
However, over the course of 72 hours, the drainage decreased significantly. Total parenteral nutrition (TPN) was administered through a Peripherally Inserted Central Catheter (PICC) line to provide necessary nutrition. A push enteroscopy, which examines the small intestine, was performed, and yielded mostly normal results with no significant findings in the biopsies taken.
Following the enteroscopy and drainage through the nasogastric tube, the patient experienced mild improvement and was able to tolerate a soft diet. A repeat CT scan on the fifth day of admission showed small clusters of small intestine loops wrapped in a membrane, without any further signs of bowel obstruction (Figure 2).
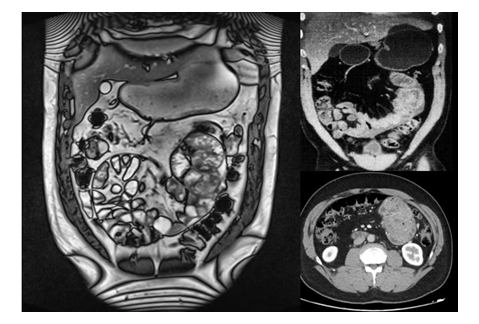
Figure 2:CT and MRI images demonstrating small intestine loops cocooned in the fibrocollagenous membrane
Due to a suspicion of encapsulating peritoneal sclerosis (EPS), a diagnostic laparoscopy was performed. At laparoscopy, the gut was found to be encapsulated by a fibrotic and inflammatory process, giving it a cocoon-like appearance (Figure 3). Multiple fibrinous adhesions and a small amount of clear fluid were observed and TB was ruled out with stains and cultures. Multiple biopsies were taken during the procedure confirming a new diagnosis of idiopathic encapsulating sclerosing peritonitis. The patient was discharged from the hospital and received ambulatory TPN for 12 hours a day, along with 200-300 ml of liquid food per day.
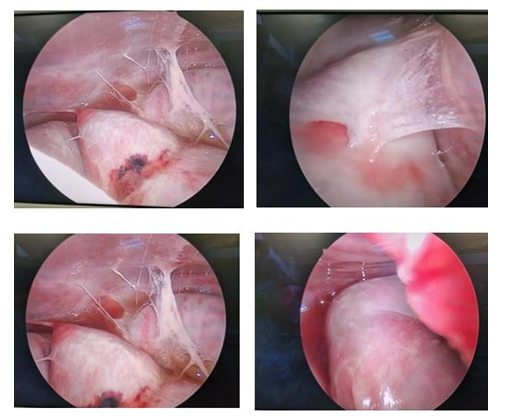
Figure 3:A series of images taken during laparoscopy demonstrating the fibrotic capsule and adhesions
The patient was then evaluated by the Autoimmune Clinic and a trial of corticosteroids followed by tamoxifen was prescribed. After 8 weeks of medical therapy, there was no clinical improvement.
He was referred to a tertiary, nationally commissioned center for EPS surgery, for definitive intervention as recommended by the multi-disciplinary team, on a semi-elective basis. After full informed consent, he underwent surgical enterolysis. Based on the departmental protocol, the abdomen was entered extra peritoneally, exposing the entire abdominal cocoon (Figure 4). With careful dissection, the cocoon was entered, dissecting off the membrane from the surface of the gut (Figure 4). The entire small bowel from the duodeno-jejunal flexure to the ileocecal junction was released without any enterotomies. At the end of the procedure, there were no obstructed areas and normal peristalsis. The abdomen was managed as a laparostomy for 48 hours with negative pressure wound therapy (set at 100mm Hg). He then underwent a relook procedure and the entire gut was found to be intact and healthy without any enterotomy or abdominal soiling. The abdomen was lavage and then underwent delayed primary closure. He made an uneventful recovery, with weaning of parenteral nutrition and re-establishment of full enteral nutrition. He was discharged home after 10 days and continues to be well and asymptomatic on a normal oral diet. He has been commenced on Tamoxifen 10mg daily for 6 months as a prophylactic antifibrotic agent to minimize recurrence risk.
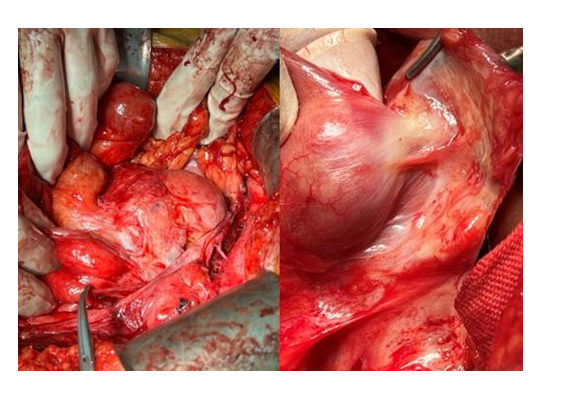
Figure 4:. Intrabdominal finding of a cocoon within a cocoon and a fibrocollagenous membrane with neovascularisation being dissected off the gut surface
Discussion
Patients with sclerosing conditions of the peritoneum pose significant dilemmas in the definitive diagnosis and management of the condition due to its rarity, heterogenicity in presentation and limited experience by clinicians with the condition. The largest number of patients in this relatively rare entity are seen in longterm peritoneal dialysis, where the sclerosis is secondary to the direct effect of peritoneal dialysis fluid.
Primary or idiopathic encapsulating peritoneal sclerosis is a condition where peritoneal sclerosis and the associated clinical manifestations are seen without any underlying precipitating or inflammatory processes. It has mainly been reported in the tropical or subtropical regions with a 2:1 male-to- female ratio.
This report highlights the complexity of diagnosing and managing encapsulating peritoneal sclerosis. While this case reports the rare primary entity, the principles of diagnosis and management are the same in both the primary and secondary varieties. Good outcomes depend on early recognition and a careful considered management plan. While further research is required to enhance our understanding of the aetiology, and medical therapies and long-term outcomes of this condition, this is hampered by small numbers and rarity. Surgical enterolysis remains the mainstay of treatment, with careful excision of the fibro collagenous obstructing membrane and thickened peritoneum
Statement of Consent
Written informed consent was obtained from the patient for the publication of this case report, including accompanying images.
References
1. Danford CJ, Lin SC, Smith MP, Wolf JL. Encapsulating peritoneal sclerosis [Internet]. World journal of gastroenterology. U.S. National Library of Medicine; [cited 2023 Jun 24]. Available from: https:// pubmed.ncbi.nlm.nih.gov/30065556/
2. Brown EA, Bargman J, van Biesen W, Chang MY, Finkelstein FO, Hurst H, et al. Length of Time on Peritoneal Dialysis and Encapsulating Peritoneal Sclerosis - Position Paper for ISPD: 2017 Update. Perit Dial Int 2017; 37: 362-374 [PMID: 28676507 DOI: 10.3747/ pdi.2017.00018]
3. Eltringham WK, Espiner HJ, Windsor CW, Griffiths DA, Davies JD, Baddeley H, et al. Sclerosing peritonitis due to practolol: a report on 9 cases and their surgical management. Br J Surg. 1977; 64: 229-235 [PMID: 856375 DOI: 10.1002/bjs.1800640402]
4. Sarker S, Kodali S, Weber F. A new meaning to butterflies in the stomach. Gastroenterology. 2015; 148: e12-e13 [PMID: 25450077 DOI: 10.1053/j.gastro.2014.07.037]
5. Sachdev A, Usatoff V, Thaow C. Sclerosing encapsulating peritonitis and methotrexate. Aust N Z J Obstet Gynaecol. 2006; 46: 58- 59 [PMID: 16441697 DOI: 10.1111/j.1479- 828X.2006.00517.x]
6. Nauen DW, Martin A, Katz A, Cohen D, Ranganathan S. A case of luteinizing thecoma with sclerosing peritonitis: revisiting a link with anti-epileptic drugs. Pediatr Blood Cancer. 2010; 54: 470-472 [PMID: 19847882 DOI: 10.1002/pbc.22325]
7. Li N, Zhu W, Li Y, Gong J, Gu L, Li M, et al. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg. 2014; 38: 1860- 1867 [PMID: 24519587 DOI: 10.1007/s00268-014-2458-6]
8. Braun N, Alscher DM, Fritz P, Edenhofer I, Kimmel M, Gaspert A, et al. Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol Dial Transplant. 2011; 26: 1033-1041 [PMID: 20709739 DOI: 10.1093/ndt/gfq488]
9. Bansal S, Sheth H, Siddiqui N, Bender FH, Johnston JR, Piraino B. Incidence of encapsulating peritoneal sclerosis at a single U.S. university center. Adv Perit Dial. 2010; 26: 75-81
10. Tannoury J, Abboud B. Idiopathic sclerosing encapsulating peritonitis: Abdominal cocoon [Internet]. World journal of gastroenterology. U.S. National Library of Medicine; [cited 2023 Jun 24]. Available from: https://pubmed.ncbi.nlm.nih.gov/22563185/
11. Moriles KE, Muhammad HF. Incidence and outcomes of encapsulating peritoneal sclerosis (EPS) and factors associated with severe EPS [Internet]. PloS one. U.S. National Library of Medicine; [cited 2023 Jun 24]. Available from: https://pubmed.ncbi.nlm.nih. gov/29293548
Received: March 6, 2023;
Accepted: March 29, 2023;
Published: March 31, 2023.
To cite this article : Nightingale K, Birtles L, Greenwood-Morgan M, Nissan A, van Dellen D, Moinuddin Z, et al. The Diagnosis and Management of a patient with Idiopathic Encapsulating Sclerosing Peritonitis. British Journal of Gastroenterology. 2023; 5(1): 286-289. doi: 10.31488/ bjg.1000138.
© The Author(s) 2023.
