Review Article / Open Access
DOI:10.31488/bjg.1000126
What Role does the Gastrointestinal Tract Microbiota Play in the Infection and Treatment of Clostridium difficile?
TM Cock
1. University of Exeter Medical School, UK
Abstract
Clostridium difficile is a spore-forming bacterium responsible for antibiotic-associated diarrhoea and Clostridium difficile infections at a great cost to the National Health Service. The human gastrointestinal tract microbiota provides a varied ecosystem supplying robust foundations for human health. In the past decade increased understanding of the gastrointestinal tract microbial makeup and its role in disease states, has prompted research endeavouring to assess the microbiota as a target for therapeutic intervention. One of the treatments widely researched within this field is Fecal Microbiota Transplantation, which is implemented with the curative aim of restoring this complex microbial eco-system.
Introduction
Initially isolated from the stool of healthy neonates, Clostridium difficile is a gram-positive, spore-forming, obligate anaerobic bacillus [1]. Found within the mammalian gastrointestinal tract (GIT), it has the ability to create toxin-mediated Clostridium difficile infections (CDI), with symptoms ranging from mild diarrhoea to pseudomembranous colitis and death [2]. Data from the US and Europe has revealed that the incidence of CDI has surpassed that of MRSA, making it the most prevalent health-care associated infection [3]. Unfortunately, in 27% of CDI cases, current standard treatments, including metronidazole and vancomycin, do not effectively treat infection or prevent recurrence [4]. This is widely due to antibiotics triggering alterations and impairment to the human GIT microbiota [5]. Faecal microbiota transplant (FMT) is a highly cost-effective alternative therapy showing promise in the treatment of CDI [6]. This review aims to assess the contribution of the GIT microbiota in CDI and its treatment. This will be done by explaining the composition of the GIT microbiome, how this contributes to the germination of Clostridium difficile and evaluating the advantages and setbacks of implementing FMT on a wider scale.
Methods
Literature was identified through searches on the electronic database PubMed, from 2010 to 2022. The key words searched for included: ‘Clostridium difficile’, ‘CDI’, ‘GIT microbiota’, ‘faecal microbiota transplant’, and ‘FMT.’ See figure 1 for the inclusion and exclusion criteria.
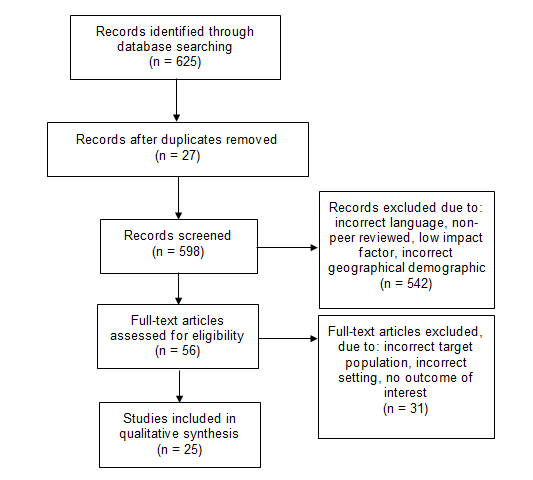
Figure 1.The inclusion and exclusion criteria for the literature selected
Discussion
The gastrointestinal tract microbiota
The GIT microbiota is an exceptionally complex ecosystem, comprised of thousands of bacteria species. In healthy adults, this is dominated by the phyla Bacteroidetes and Firmicutes. These bacteria play a crucial role in host metabolism, nutrition function, maturation of the immune system and protection against pathogens. Various factors throughout a person’s life influence the composition of their GIT microbiota, including: diet, geography, antibiotic use and disease [7]. Disturbance to the GIT microbiota, known as intestinal dysbiosis, can significantly impact the structure and function of its bacteria. Such changes to the indigenous microbial composition disrupt colonisation resistance, favouring infection, and germination of Clostridium difficile [8].
Susceptibility to CDI is strongly associated with antibiotic use, due to their influence on the GIT microbiota, with Cephalosporins, Penicillin and Fluoroquinolones implicated [9]. Different studies have assessed such effects of antibiotics describing the microbial composition of patients with CDI, observing similar results in relation to a reduction in species richness. In contrast to healthy controls, patients with CDI showed an increase in Firmicutes and Proteobacteria phyla and a decrease in Bacteroidetes phylum disrupting normal function of the microbiota. The data from these studies is highly significant, not only due to their similar reproduceable results, but also because they are published in peer review journals with a high impact factor [10,11].
Bile-acid mediated germination
Bile-acids are the end products of cholesterol metabolism in the liver, they play a pivotal role in mediating Clostridium difficile spore germination [12]. In humans, there are two main primary bile-acids: cholic acid and chenodeoxycholic acid. The majority of cholic acid and chenodeoxycholic acid secreted into the gut is reabsorbed by the liver, however some bile-acids evade hepatic recirculation. These enter the large intestine where the microbiota act upon them, altering their composition [13]. In patients with an altered GIT microbiota the numbers of bacteria producing hydrolytic enzymes are reduced triggering a reduction in the secondary bile-acids responsible for the inhibition of vegetative cell growth. Simultaneously, the volume of cholic acid and chenodeoxycholic acid will dramatically rise, stimulating spore germination, resulting in CDI. Additionally, depletion of the GIT microbiota produces an excess supply of nutrients supporting germination of Clostridium difficile [7]. In two studies, administration of antibiotics resulted in a shift within the bile acid pool thereby increasing spore germination. Both studies utilised a Murine model, showing replicable results [14].
Conventional treatment of CDI
Currently, the mainstay in the treatment of CDI is oral metronidazole or vancomycin for 10-14 days, in the instance of mild or moderate disease, alongside cessation of the antibiotic therapy which may have predisposed the patient to infection. In patients suffering with severe CDI, oral vancomycin (± IV metronidazole) or oral fidaxomicin are recommended. Between 20% and 30% of patients with CDI fail these initial treatments and of these up to 60% experience a second CDI [16]. The majority of recurrences arise due to the original strain rather than re-infection with an alternate strain [17]. However, resistance to vancomycin and metronidazole are not deemed a factor in recurrence, instead it is thought that these antibiotics contribute to a continued dysbiosis, disturbing the body’s natural colonisation resistance [18]. Rising instance of health care associated infections in the United Kingdom are estimated to cost the National Health Service £1 billion annually [19], placing the trial and implementation of alternative treatments high on the agenda.
Faecal microbiota transplantation
Faecal microbiota transplantation (FMT) involves the infusion of faecal suspension garnered from a healthy donor, with the curative aim of restoring a healthy gut microbiota in a patient with CDI [17]. The rationale underpinning this procedure is based upon re-establishing the specific commensal species within the GIT, depleted by antibiotic treatments. This will allow them to resume normal functioning, suppressing pathogen proliferation. In particular FMT aims to re-instate Butyrate-producing bacteria which are depleted in CDI. Butyrate - a short-chain fatty acid – is crucial in energy production, intestinal epithelial cell homeostasis, immune function and normal microbial growth. This is significant as recurrent CDI is strongly associated with a reduction in Butyrate-producing bacteria. Re-implanting strains of Butyrate-producing bacteria via FMT can restore normal microbial biodiversity and metabolic function. Furthermore, FMT significantly increases the amount of secondary bile acids, restoring short-chain fatty acid production leading to greater inhibition of vegetative cell growth [20].
The optimum method of administration has not yet been determined. However, one pooled analysis indicated that use of gastroscopy, nasogastric tube, or nasojejunal tube are marginally less efficacious when compared with rectal tube/enema and colonoscopy. Colonoscopic application was shown to have a moderately greater curative effect than nasogastric application; however this was not statistically significant (93% vs 85%) [21].
FMT predominantly utilises stool samples from related donors or intimate partners and screening protocols are requisite for minimising infection transmission. Donor screening is extensive including a full patient history alongside the following lab tests: full blood count, liver function tests, screening for hepatitis A, B and C, human immunodeficiency virus I and II, human T-cell lymphotropic virus, Cytomegalovirus, Epstein-Barr virus, syphilis, stool tests for Clostridium difficile toxin A and B, stool microscopy for ova cysts and parasites and selective stool culture [17]. This is a time-consuming process, limiting availability of viable stool samples. However, a recent study demonstrated no substantial difference in the efficacy of CDI treatment via FMT when using fresh versus frozen samples or inpatient identified donors versus standardised banked faecal matter [11]. The use of oral frozen faecal capsules from bank donors in the treatment of CDI, has been trialled with substantial clinical efficacy and resolution of symptoms in 90% of cases. The use of such capsules will streamline the FMT procedure, rendering it more accessible and “palatable” for patients [17].
FMT is the only therapy utilised in the treatment of CDI that restores the biodiversity of the GIT microbiota without extending the perturbation of the normal microbial eco-system. Numerous studies have reinforced the safety and efficacy of FMT in the treatment of CDI, it is particularly advocated for in patients with recurrence of disease following conventional treatment [22]. Additionally, there is extensive research supporting the efficacy of FMT in severe CDI. One study suggested the implementation of FMT in moderate CDI not responding to vancomycin, for at least a week and severe CDI with no response to antibiotics after 48 hours [23].
Another measure of clinical effectiveness is Quality Adjusted Life Years (QALYs), a single figure accounting for the length and quality of life an intervention provides. The QALYs of FMT (0.242) versus conventional treatment (0.235) for CDI have been compared revealing FMT to be more cost-effective at $1,699 compared with oral vancomycin at $3,788. The QALY figures utilised in this review were directly sourced from studies and therefore results could reflect altered inputs and presumptions [6].
Despite mounting research supporting implementation of FMT in patients with CDI many clinicians remain concerned that the lack of “palatability” remains an evident barrier, deterring patients from the procedure. Nevertheless, patient acceptance does not seem to be a major factor prohibiting wider use of FMT, this was demonstrated in a small survey of 77 patients. These participants had all recently undergone colonoscopic FMT for recurrent CDI, 53% expressed that they would elect to undergo FMT as their preferred primary treatment in the case of future recurrence [24]. Whilst this survey presents evidence that public perception of FMT may not impact upon the success of wider application of FMT, it provides insight into the views of a relatively small sample size. This limitation makes it difficult to determine if the outcome is a true reflection of the opinions of the public.
Furthermore, concerns have been raised over the efficacy and safety of performing FMT on patients who are immuno-compromised and are particularly at risk of CDI. While application within this patient group has been utilised, implementation has been limited due to worry due to theoretic potential for bacterial translocation and infection. Though the evidence base regarding the use of FMT in immune-compromised patients is expanding no clinical trials exist to date. Nonetheless, current literature favours the use of FMT in immuno-compromised patients, with an acceptable adverse effect profile and negligible risk of adverse events [25].
Conclusion
The GIT microbiota plays a crucial role in CDI making it a valuable target for therapeutic intervention. A healthy, heterogeneous microbial composition offers natural pathogen resistance and inhibition of spore germination aiding in the prevention of CDI. Succeeding antibiotic use, the GIT microbiota is depleted lacking variation, the loss of many secondary bile acids and Butyrate-producing bacteria contributes the ideal environment for Clostridium difficile colonisation, germination and infection. The conventional standard of treatment against CDI is ineffectual and often results in secondary infection due to continued dysbiosis. In addition, the burden of health care acquired infections on the National Health Service is rising and therefore finding an alternative treatment with high efficacy and low cost is paramount. FMT provides a promising, cost-effective, alternative therapy and is currently the only available intervention that restores the diversity and species richness of GIT microbiota allowing re-establishment of the patient’s natural colonisation resistance. The use of frozen faecal capsules will streamline this procedure, vastly increasing accessibility. While more research is needed to attain a greater understanding of public perception of these capsules, they offer promise due to their superior “palatability”. In addition, further research needs to be undertaken to assess the efficacy and safety of FMT and the frozen faecal capsules in patients who are immuno-compromised.
References
1. Giel JL, Sorg JA, Sonenshein AL, et al. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One. 2010 Jan 15;5(1):e8740. doi: 10.1371/journal.pone.0008740.
2. Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clinical Infectious Diseases. 2012 Aug;55 Suppl 2(Suppl 2):S65-70. doi: 10.1093/cid/cis319.
3. Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. Journal of Pharmacy Practice. 2013 Oct;26(5):464-75. doi: 10.1177/0897190013499521.
4. Chaparro-Rojas F, Mullane KM. Emerging therapies for Clostridium difficile infection - focus on fidaxomicin. Infection and Drug Resistance. 2013 Jun 28;6:41-53. doi: 10.2147/IDR.S24434.
5. Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016 Nov;65(11):1906-1915. doi: 10.1136/gutjnl-2016-312297. Epub 2016 Aug 16. PMID: 27531828.
6. Arbel LT, Hsu E, McNally K. Cost-Effectiveness of Fecal Microbiota Transplantation in the Treatment of Recurrent Clostridium difficile Infection: A Literature Review. Cureus. 2017 Aug 23;9(8):e1599. doi: 10.7759/cureus.1599.
7. Schäffler H, Breitrück A. Clostridium difficile - From Colonization to Infection. Frontiers in Microbiol. 2018 Apr 10;9:646. doi: 10.3389/fmicb.2018.00646.
8. Robinson CJ, Young VB. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Micr. 2010 Jul;1(4):279-284. doi: 10.4161/gmic.1.4.12614. Epub 2010 May 24. PMID: 20953272; PMCID: PMC2954510.
9. Manges AR, Labbe A, Loo VG, et al. Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J Infect Dis. 2010 Dec 15;202(12):1877-84. doi: 10.1086/657319.
10. Antharam VC, Li EC, Ishmael A, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013 Sep;51(9):2884-92. doi: 10.1128/JCM.00845-13.
11. Hamilton MJ, Weingarden AR, Unno T, et al. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013 Mar-Apr;4(2):125-35. doi: 10.4161/gmic.23571.
12. Howerton A, Ramirez N, Abel-Santos E. Mapping interactions between germinants and Clostridium difficile spores. Journal of Bacteriology. 2011 Jan;193(1):274-82. doi: 10.1128/JB.00980-10.
13. Zhu D, Sorg JA, Sun X. Clostridioides difficile Biology: Sporulation, Germination, and Corresponding Therapies for C. difficile Infection. Frontiers in Cellular and Infection Microbiology. 2018 Feb 8;8:29. doi: 10.3389/fcimb.2018.00029.
14. Antunes LC, Finlay BB. A comparative analysis of the effect of antibiotic treatment and enteric infection on intestinal homeostasis. Gut Microbes. 2011 Mar-Apr;2(2):105-8. doi: 10.4161/gmic.2.2.15610.
15. Giel JL, Sorg JA, Sonenshein AL, Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One. 2010 Jan 15;5(1):e8740. doi: 10.1371/journal.pone.0008740.
16. Hopkins RJ, Wilson RB. Treatment of recurrent Clostridium difficile colitis: a narrative review. Gastroenterology Report. 2018 Feb;6(1):21-28. doi: 10.1093/gastro/gox041
17. Ofosu A. Clostridium difficile infection: a review of current and emerging therapies. Annals of Gastroenterology. 2016 Apr-Jun;29(2):147-54. doi: 10.20524/aog.2016.0006
18. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. The New England J Med. 2015 Feb 26;372(9):825-34. doi: 10.1056/NEJMoa1408913.
19. Agha M. Epidemiology and Pathogenesis of C. difficile and MRSA in the Light of Current NHS Control Policies: A Policy review. Annals of Med Surg. 2012 Oct 6;1:39-43. doi: 10.1016/S2049-0801(12)70012-2.
20. Carlucci C, Petrof EO, Allen-Vercoe E. Fecal Microbiota-based Therapeutics for Recurrent Clostridium difficile Infection, Ulcerative Colitis and Obesity. EBioMedicine. 2016 Nov;13:37-45. doi: 10.1016/j.ebiom.2016.09.029.
21. Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection. 2012 Dec;40(6):643-8. doi: 10.1007/s15010-012-0307-9.
22. Drekonja D, Reich J, Gezahegn S, et al. Fecal Microbiota Transplantation for Clostridium difficile Infection: A Systematic Review. Annals of Internal Med. 2015 May 5;162(9):630-8. doi: 10.7326/M14-2693.
23. Bakken JS, Borody T, Brandt LJ, et al. Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011 Dec;9(12):1044-9. doi: 10.1016/j.cgh.2011.08.014.
24. Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. The American J Gastroenterol. 2012 Jul;107(7):1079-87. doi: 10.1038/ajg.2012.60.
25. Abu-Sbeih H, Ali FS, Wang Y. Clinical Review on the Utility of Fecal Microbiota Transplantation in Immunocompromised Patients. Current Gastroenterol Reports. 2019;21(8). doi: 10.1007/s11894-019-0677-6
Received: February 04, 2022;
Accepted: February 24, 2022;
Published: February 28, 2022.
To cite this article : TM Cock. What Role does the Gastrointestinal Tract Microbiota Play in the Infection and Treatment of Clostridium difficile?. British Journal of Gastroenterology. 2022; 4(1): 225- 228. doi: 10.31488/ bjg.1000126.
© TM Cock. 2022.
